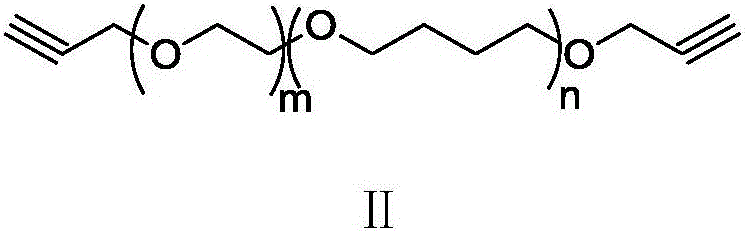
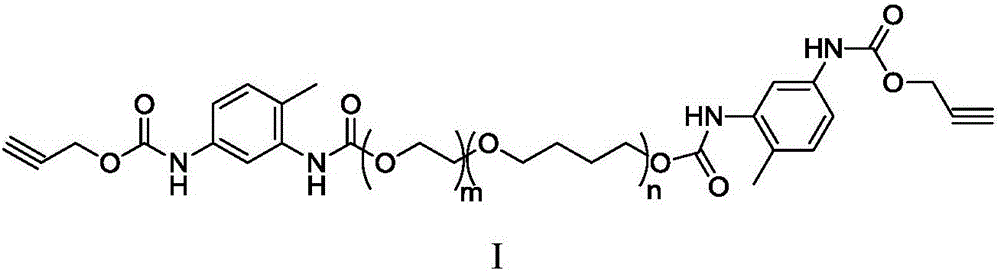
Acetylene-terminated ethylene oxide tetrahydrofuran copolyether containing carbamic acid ester units and synthesis method thereof
A technology of alkynyl-terminated oxirane tetrahydrofuran copolyether and hydroxyl-terminated oxirane tetrahydrofuran copolyether, which is applied in the field of alkynyl-terminated oxirane tetrahydrofuran copolyether and its synthesis, and can solve the problem of polytriazole fracture elongation Low-level problems, to achieve the effect of improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Synthesis of propargyl (3-isocyanato-4-methyl) phenyl carbamate
[0018] Under nitrogen protection, 5.6 g (0.1 mol) of propynyl alcohol was added dropwise to a mixture of 52.2 g (0.3 mol) of toluene-2,4-diisocyanate and 100 mL of toluene, and the temperature was kept at 40° C. during the dropwise addition. After the dropwise addition, react at 60°C for 6h to end the reaction. After cooling down, a solid was precipitated, filtered and dried to obtain 14 g of a white solid with a yield of 60.9%.
[0019] Characterization data:
[0020] 1 H NMR (DMSO-d 6 ,500MHz): δ=9.90(s,1H),7.31(s,1H),7.19(m,2H),4.76(d,2H,J=2.0Hz),3.56(t,1H,J=2.0Hz) ,2.23(s,3H); 13 C NMR (DMSO-d 6 ,125MHz): δ=153.0,138.2,132.3,131.3,127.2,124.6,116.4,114.9,79.3,78.0,52.5,17.6.
[0021] IR(KBr,cm -1 ):ν=3331, 3294, 2281, 2124, 1714, 1623, 1553, 1516, 1388, 1314, 1283, 1227, 1075, 916;
[0022] Elemental Analysis: C 12 h 10 N 2 o 3 , theoretical value C 62.6, H 4.38, N 12.17; calculated v...
Embodiment 2
[0031] (1) Synthesis of propargyl (3-isocyanato-4-methyl) phenyl carbamate
[0032] Under nitrogen protection, 5.6 g (0.1 mol) propynyl alcohol was added dropwise to a mixture of 69.6 g (0.4 mol) toluene-2,4-diisocyanate and 100 mL toluene, and the temperature was kept at 50° C. during the dropwise addition. After the dropwise addition, react at 60°C for 6h to end the reaction. After cooling down, a solid was precipitated, filtered and dried to obtain 14.2 g of a white solid, with a yield of 62.0%.
[0033] (2) Synthesis of tetrahydrofuran copolyether containing carbamate unit-terminated alkynyl oxirane tetrahydrofuran
[0034] Under nitrogen protection, 15.3g (66.6mmol) propargyl (3-isocyanato-4-methyl) phenyl carbamate, 100g hydroxyl-terminated oxirane tetrahydrofuran block copolyether (66.6mmol OH) and Add 500mL of dry toluene to the reaction flask. After stirring at 70°C for 24 hours, the reaction was terminated, and the toluene was concentrated to drive off to obtain 1...
Embodiment 3
[0036] (1) Synthesis of propargyl (3-isocyanato-4-methyl) phenyl carbamate
[0037] Under nitrogen protection, 5.6 g (0.1 mol) propynyl alcohol was added dropwise to a mixture of 87 g (0.5 mol) toluene-2,4-diisocyanate and 100 mL toluene, and the temperature was kept at 50° C. during the dropwise addition. After the dropwise addition, react at 70° C. for 5 h to end the reaction. After cooling down, a solid was precipitated, filtered and dried to obtain 15.6 g of a white solid with a yield of 68.1%.
[0038] (2) Synthesis of tetrahydrofuran copolyether containing carbamate unit-terminated alkynyl oxirane tetrahydrofuran
[0039] Under nitrogen protection, 1.15g (5mmol) propargyl (3-isocyanato-4-methyl) phenyl carbamate, 11.5g hydroxyl-terminated oxirane tetrahydrofuran random copolymer ether (5mmol OH) and 100mL Add dry toluene to the reaction flask. After stirring at 60°C for 24 hours, the reaction was terminated, and the toluene was removed by concentration to obtain 11.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
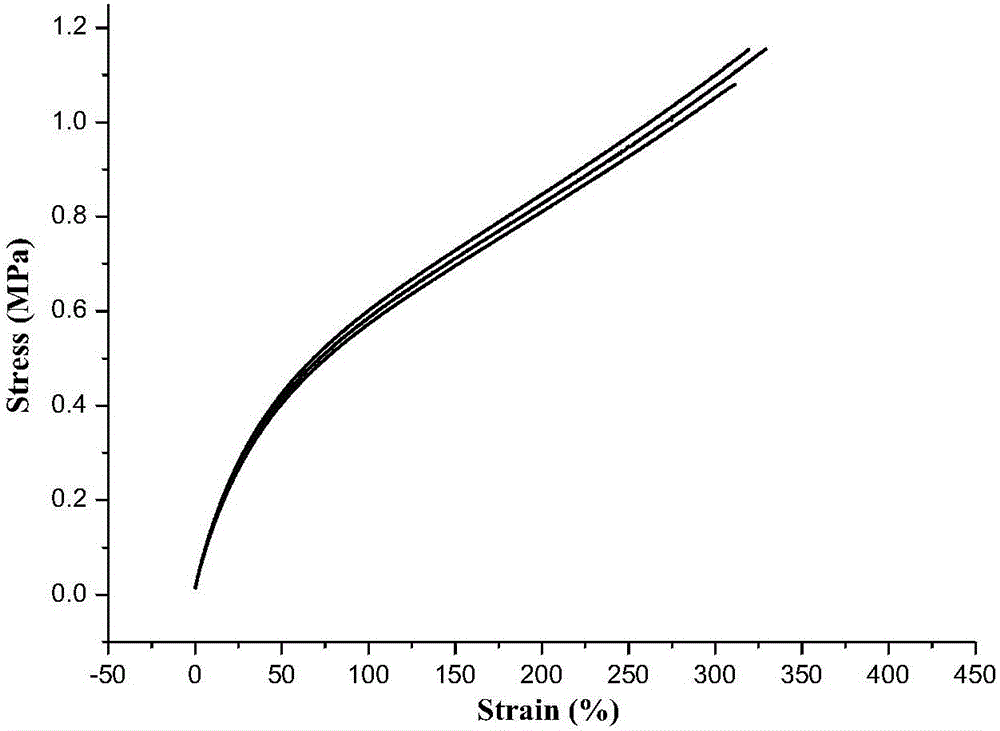
| Maximum stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com