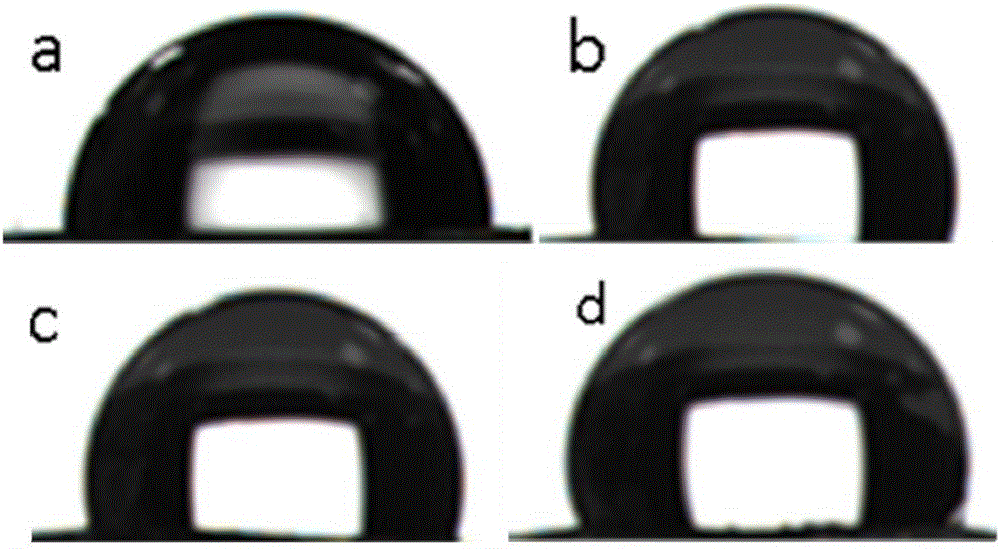
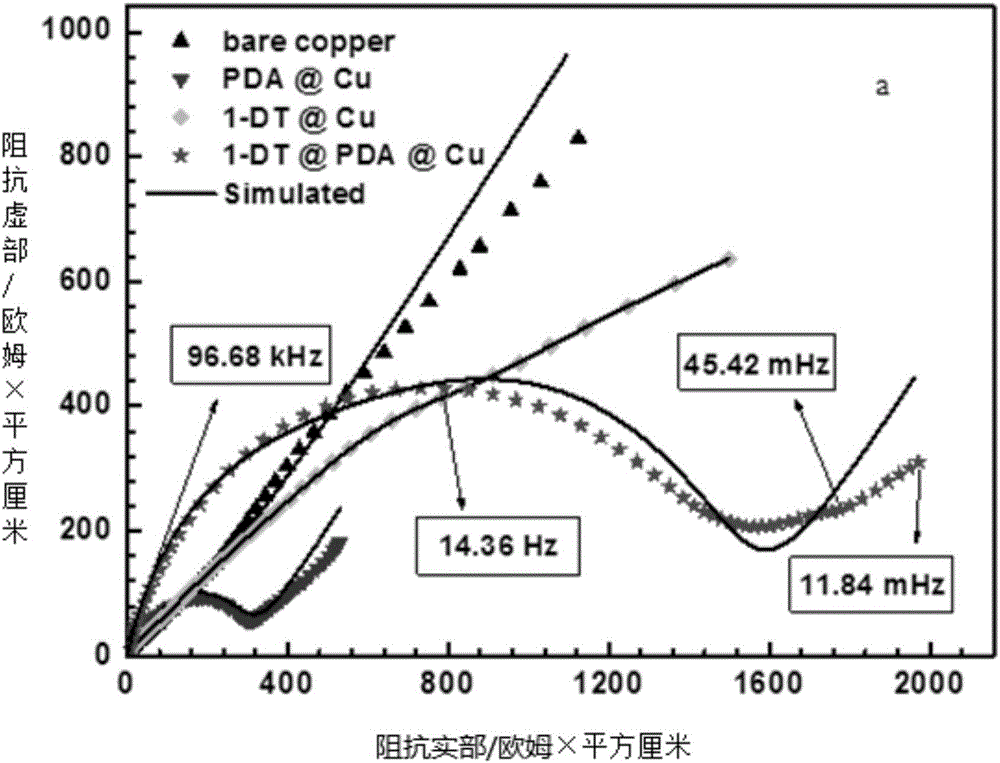
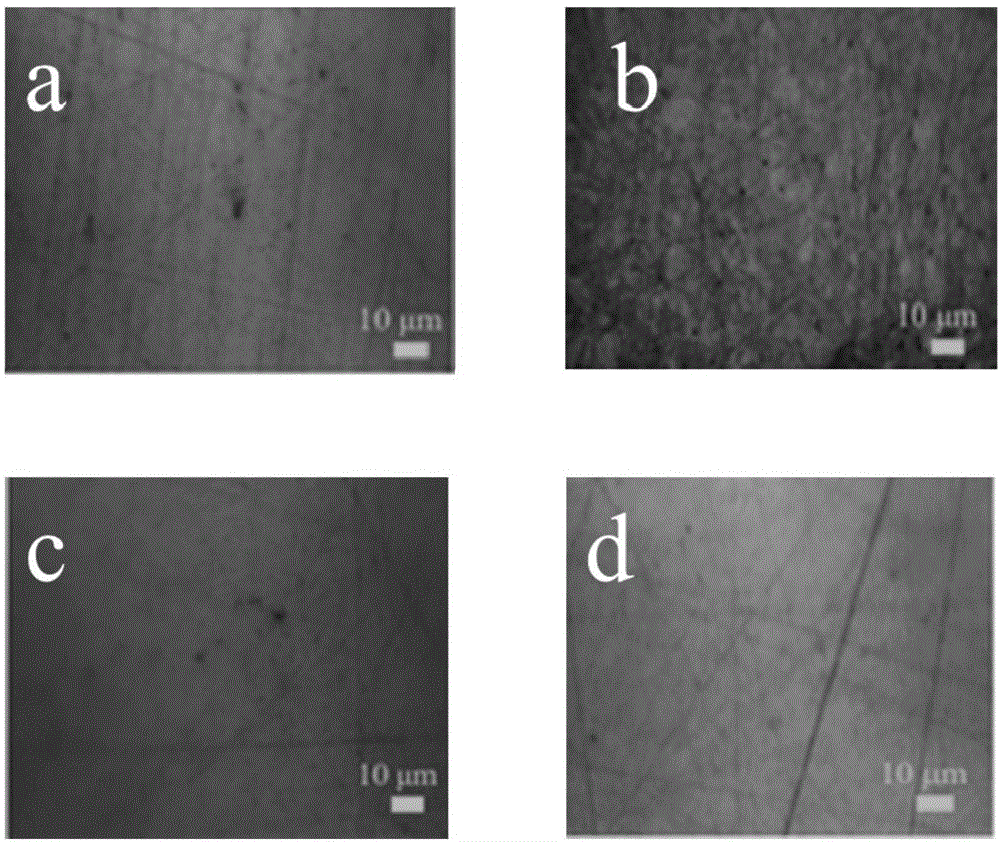
Preparation method for corrosion-resistant hydrophobic film on metal surface
A metal surface, corrosion-resistant technology, applied in the direction of metal material coating process, coating, etc., can solve the problem that copper corrosion cannot be well inhibited, and achieve the effect of simple method, short time consumption and reduced loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Copper pretreatment: Grind the electrode on coarse sandpaper and fine sandpaper respectively to remove copper oxide on the electrode surface, then ultrasonically clean it in water, ethanol and water for 8-10 minutes, and then polish it with 0.5 micron aluminum oxide , after the copper surface forms a bright mirror surface, ultrasonically clean it again in water, ethanol and water for 8-10 minutes;
[0032] (2) Rapidly immerse the copper electrode obtained in (1) into 7M HNO 3 Soak in the solution for 10-15 seconds to remove the copper oxide formed on the copper surface during the pretreatment process, and then rinse with water and ethanol.
[0033] (3) Preparation of hydrophobic film on copper surface: immerse the above-mentioned treated copper electrode in a Tris-HCl (pH 8.5) buffer solution of 0.5 mg / mL dopamine, take it out after 2 hours and wash the remaining molecules on the surface that do not interact with copper , Then immerse the electrode in 5mM dodecyl m...
Embodiment 2
[0035] (1) Copper pretreatment: grind the electrode on coarse sandpaper and fine sandpaper respectively, remove the copper oxide on the electrode surface, then successively ultrasonically clean in water, ethanol and water for 8 minutes, then polish with 0.5 micron aluminum oxide, wait After forming a bright mirror surface on the copper surface, ultrasonically clean it again in water, ethanol and water for 8 minutes;
[0036] (2) Rapidly immerse the copper electrode obtained in (1) into 7M HNO 3 Soak in the solution for 10 seconds to remove the copper oxide generated on the copper surface during the pretreatment process, and then rinse with water and ethanol.
[0037] (3) Preparation of hydrophobic film on copper surface: immerse the above-mentioned treated copper electrode in a Tris-HCl (pH8.5) buffer solution of 0.5 mg / mL dopamine, take it out after 1 hour and rinse the remaining surface with water that does not interact with copper. Molecules, then immerse the electrode in ...
Embodiment 3
[0043] (1) Copper pretreatment: grind the electrode on coarse sandpaper and fine sandpaper respectively, remove the copper oxide on the electrode surface, then successively ultrasonically clean in water, ethanol and water for 8 minutes, then polish with 0.5 micron aluminum oxide, wait After forming a bright mirror surface on the copper surface, ultrasonically clean it again in water, ethanol and water for 8 minutes;
[0044] (2) Rapidly immerse the copper electrode obtained in (1) into 7M HNO 3 Soak in the solution for 10 seconds to remove the copper oxide generated on the copper surface during the pretreatment process, and then rinse with water and ethanol.
[0045] (3) Preparation of hydrophobic film on copper surface: immerse the above-mentioned treated copper electrode in Tris-HCl (pH 9) buffer solution of 0.3mg / mL dopamine, take it out after 1h and wash the remaining molecules on the surface that do not interact with copper , Then immerse the electrode in 4mM dodecyl mer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com