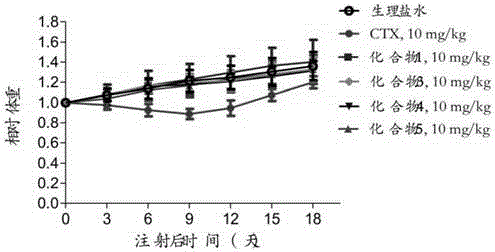
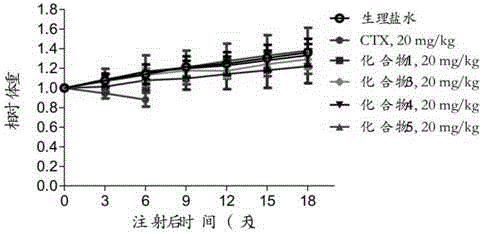
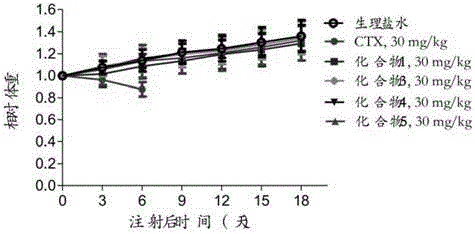
Preparation method and application of cabazitaxel prodrug
A cabazitaxel and drug technology, applied in the field of cabazitaxel drug precursor and its preparation, can solve the problems of poor drug stability, poor water solubility of cabazitaxel, low maximum tolerated dose, etc. In vitro and in vivo stability, the effect of avoiding the use of formulation excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Example 1 Synthesis of CTX prodrug 1 containing unsaturated alkane chain compound
[0042] Add CTX (100mg, 0.12mmol) and cis-4,7,10,13,16,19-docosahexaenoic acid (DHA, 39mg, 0.12mmol) into a 100mL round bottom flask, dissolve in 5mL anhydrous In DCM (dichloromethane), add EDC·HCl (25 mg, 0.13 mmol), DMAP (4-dimethylaminopyridine) (16 mg, 0.13 mmol) and DIEA (N,N-diisopropylethylamine) ( 21 μL, 0.13 mmol). Stir overnight at 25°C, then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine respectively; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate, and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (DCM:MeOH=200:1) yielded product 1 (80 mg, yield 58%).
[0043] product 1 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0044] 1 H NMR (400MHz, CDCl3): δ0.86-0.90(t, 3H), 1.20-1.22(d, 6H, J=6.4), 1.25(s, 3H), 1.35(s, 9H), 1.57-1.63(...
Embodiment 2
[0046] Example 2 Synthesis of CTX prodrug 2 containing unsaturated alkane chain compound
[0047] Add CTX (100mg, 0.12mmol) and linolenic acid (34mg, 0.12mmol) to a 100mL round bottom flask, dissolve in 5mL of anhydrous dichloromethane, then add EDC·HCl (25mg, 0.13mmol), 4-dimethyl Aminopyridine (16 mg, 0.13 mmol) and N,N-diisopropylethylamine (21 μL, 0.13 mmol). Stir overnight at 25°C, then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine respectively; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate, and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (DCM:MeOH=200:1) to obtain product 2 (115 mg, yield 87%).
[0048] product 2 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0049] 1 H NMR (400MHz, CDCl3): δ0.86-0.90(t,3H),1.22(s,9H),1.26(s,8H),1.35(s,9H),1.51-1.55(t,2H),1.62 -1.67(m,2H),1.76-1.82(m,2H),2.00(s,3H),2.04-2.08(m,4H),...
Embodiment 3
[0051] Example 3 Synthesis of CTX prodrug 3 containing unsaturated alkane chain compound
[0052] Add CTX (100mg, 0.12mmol) and linoleic acid (34mg, 0.12mmol) into a 100mL round bottom flask, dissolve in 5mL of anhydrous dichloromethane, then add EDC·HCl (25mg, 0.13mmol), 4-di Aminopyridine (16 mg, 0.13 mmol) and N,N-diisopropylethylamine (21 μL, 0.13 mmol). Stir overnight at 25°C, then wash with 5% citric acid, saturated sodium bicarbonate, and saturated brine respectively; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate, and remove the solvent under reduced pressure; the solid is separated and purified by column chromatography (DCM:MeOH=200:1) to obtain product 3 (120 mg, yield 91%).
[0053] product 3 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0054] 1 H NMR (400MHz, CDCl 3 ):δ0.86-0.90(t,3H),1.22(s,9H),1.26(s,14H),1.35(s,9H),1.51-1.55(t,2H),1.62-1.67(m,2H ),1.76-1.82(m,2H),2.00(s,3H),2.04-2.08(m,4H),2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com