Method for high-selectivity preparation of gamma-GVL by homogeneous catalysis
A homogeneous catalysis and valerolactone technology, applied in organic chemistry and other fields, can solve problems such as time-consuming, hinder large-scale production, and unsatisfactory industrial production, and achieve convenient operation, improved safety and economy, and low equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
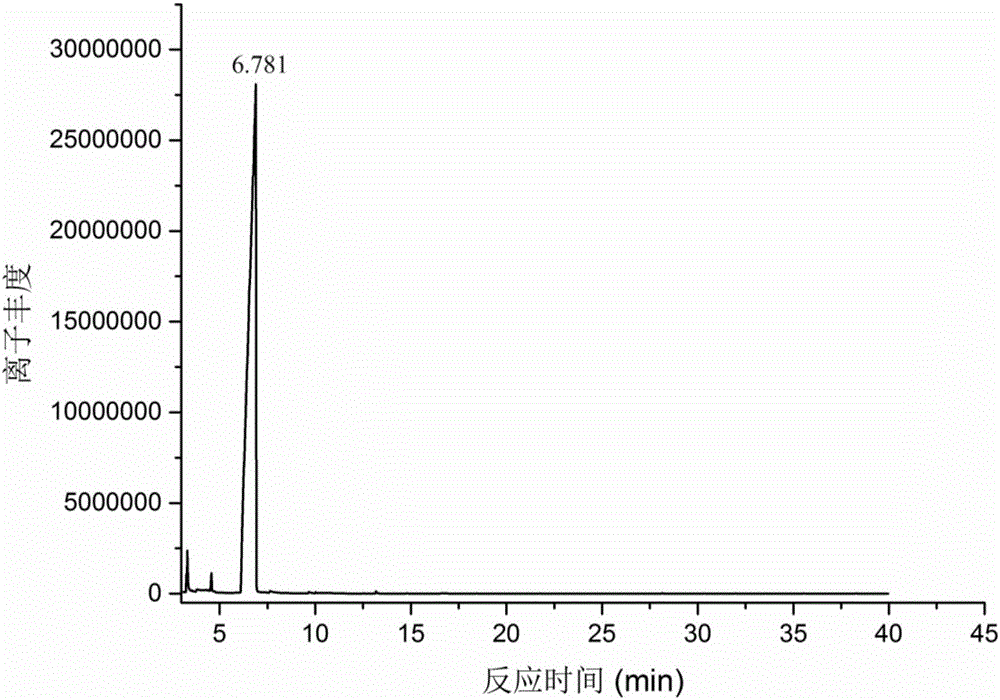
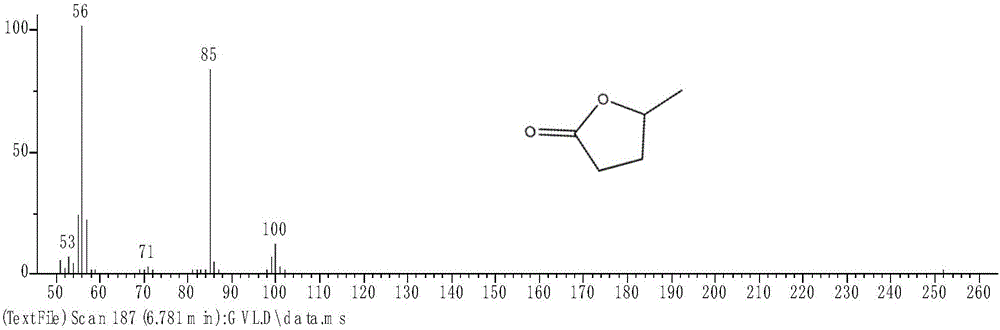
[0025] Add 0.5g methyl levulinate, 8g isopropanol, 0.25g aluminum isopropoxide to the glass reaction tube, stir evenly, add a stirrer, put it into the microwave synthesis reactor after installation; set the initial microwave power as 300W, set the temperature at 150°C, and reacted for 30 minutes under constant speed stirring. After the reaction was completed, the system air cooling was used to quickly cool down to 40°C, and then the reaction mixture was taken out. The reaction product was analyzed by gas chromatography-mass spectrometry, and the results were as follows: figure 1 and figure 2 As shown, it is confirmed that the product of this reaction is determined to be γ-valerolactone, and it also proves that the conversion rate of the substrate in this reaction process is high, and almost no side reactions occur, which is beneficial to the separation and purification of later products. It is calculated that the conversion rate of methyl levulinate is 100%, and the yield of...
Embodiment 2
[0027] Add 0.5g ethyl levulinate, 8g isopropanol, 0.25g aluminum isopropoxide to the glass reaction tube, stir evenly, add a stirrer, put it into the microwave synthesis reactor after installation; set the initial microwave power as 300W, set the temperature at 160°C, and carried out the reaction for 40 minutes under constant stirring. After the reaction was completed, the system air cooling was used to quickly cool down to 40°C, and then the reaction mixture was taken out. Calculated by gas chromatography analysis, the conversion rate of ethyl levulinate was 100%, and the yield of GVL was 88.14%.
Embodiment 3
[0029] Add 0.5g butyl levulinate, 8g isopropanol, 0.25g aluminum isopropoxide to the glass reaction tube, stir evenly, add a stirrer, put it into the microwave synthesis reactor after installation; set the initial microwave power as 300W, set the temperature at 160°C, keep the reaction conditions for 40min, and carry out the reaction under constant speed stirring. Calculated by gas chromatography analysis, the conversion rate of butyl levulinate was 100%, and the yield of GVL was 94.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com