Novel Mn<4+> activated high color purity fluoride red light emitting material preparation method
A technology of red light-emitting and light-emitting materials, applied in the fields of light-emitting materials, chemical instruments and methods, etc., can solve the problems of high color temperature, lack of spectrum in the red light region, poor color rendering index, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Weigh 0.062g of potassium hexafluoromanganate and dissolve it in 5 mL of hydrofluoric acid (40 wt %), stir at room temperature until completely dissolved, add 0.760 mL of hexafluorozirconic acid (45 wt %) aqueous solution to this solution, stir for 10 min; Then add 1.305g KF solid and continue to stir for 30 min. The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product K 3 ZrF 7 :Mn 4+ .
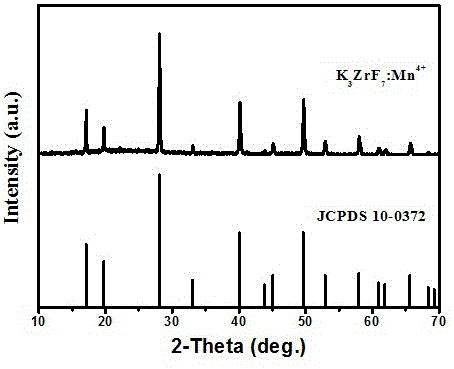
[0016] The XRD diffraction pattern of this fluorescent powder is attached figure 1 shown, with standard card JCPDS 10-0372 (K 3 ZrF 7 ) in contrast, the two are completely consistent, and no diffraction peaks of any heterogeneous phases are observed, which indicates that the samples we synthesized have a single crystal phase.
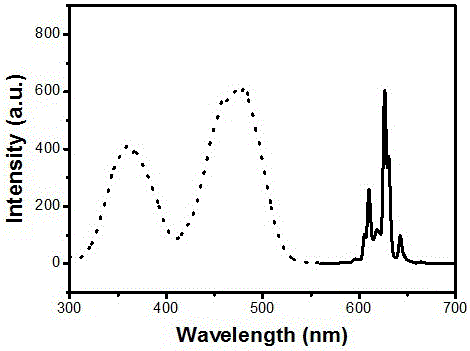
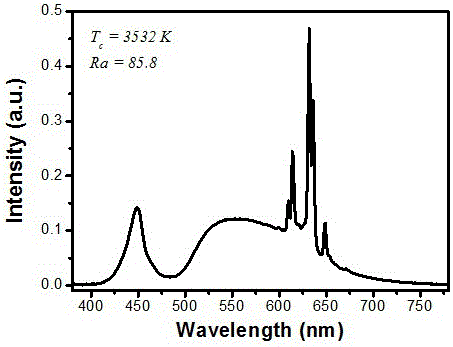
[0017] attached figure 2 Shown are the room temperature excitation spectrum (monitored at 627 nm) and emission s...
Embodiment 2
[0020] Weigh 0.031g of potassium hexafluoromanganate and dissolve it in 5 mL of hydrofluoric acid (40 wt %), stir at room temperature until completely dissolved, add 0.760 mL of hexafluorozirconic acid (45 wt %) aqueous solution to this solution, stir for 10 min; then add 1.740g of potassium fluoride solid and continue stirring for 60 min. The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product K 3 ZrF 7 :Mn 4+ .
Embodiment 3
[0022] Weigh 0.093g of potassium hexafluoromanganate and dissolve it in 10 mL of hydrofluoric acid (40 wt %), stir at room temperature until completely dissolved, add 0.760 mL of hexafluorozirconic acid (45 wt %) aqueous solution to this solution, stir for 10 min; then add 1.305g of potassium fluoride solid and continue stirring for 30 min. The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product K 3 ZrF 7 :Mn 4+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com