Use of CTLA4 compound for achieving drug-free remission in subjects with early RA
一种受试者、无药物的技术,应用在类风湿性关节炎,早期类风湿性关节炎领域,能够解决没有证实等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0134] The lyophilized CTLA4Ig (250mg / vial) drug product is a sterile, nonpyrogenic lyophile suitable for intravenous (IV) administration. Each single-use vial contains 250 mg of CTLA4Ig, which is constituted with USP Sterile Water for Injection and further diluted with USP 0.9% Sodium Chloride Injection at the time of use.
[0135] The batch recipe for the 115 liter batch size is described in Table 4 below.
[0136] Table 4
[0137] batch recipe
[0138] components Quantity (kg) CTLA4Ig drug substance a
4.6 Maltose monohydrate 9.2 hydrochloric acid Adjust to pH 7.5 sodium hydroxide Adjust to pH 7.5 Water for Injection Appropriate amount to 119.6 b
[0139] a CTLA4Ig drug substance: protein concentration 50 mg / ml, 25 mM sodium phosphate, 50 mM sodium chloride, pH 7.5, < 5% HMW substance.
[0140] b Prepared bulk solution density = approximately 1.04 g / ml.
[0141] Add the desired amount of CTLA4Ig drug substance t...
Embodiment II
[0159] CTLA4Ig SC, 125 mg / ml (125 mg / vial) drug product is formulated as a sterile, pyrogen-free ready-to-use solution suitable for subcutaneous administration. A batch of CTLA4Ig SC, 125 mg / ml (125 mg / vial) drug product was manufactured at 5-L scale (3,500 vials). The batch formulation is described in Table 7 below.
[0160] Table 7
[0161] batch recipe
[0162] components Quantity (gm) CTLA4Ig drug substance a
625 sucrose 850 Poloxamer 188 40 Sodium dihydrogen phosphate, monohydrate 0.715 Disodium hydrogen phosphate, anhydrous 4.86 Water for Injection Appropriate amount to 5.0 L Total batch size (L) 5.0
[0163] a CTLA4Ig drug substance: protein concentration 50 mg / ml, 25 mM sodium phosphate, 50 mM sodium chloride, pH 7.5, < 5% HMW substance.
[0164] As described above in Example I, the manufacturing process for the CTLA4Ig SC, 125 mg / ml (125 mg / vial) drug product included bulk drug substance from 25 mM so...
Embodiment III
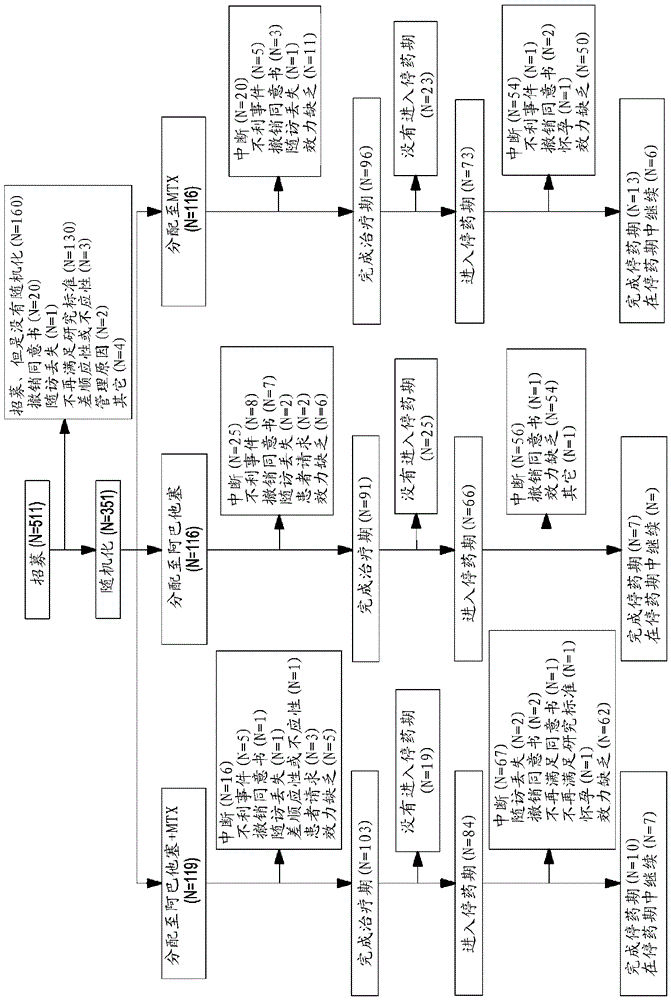
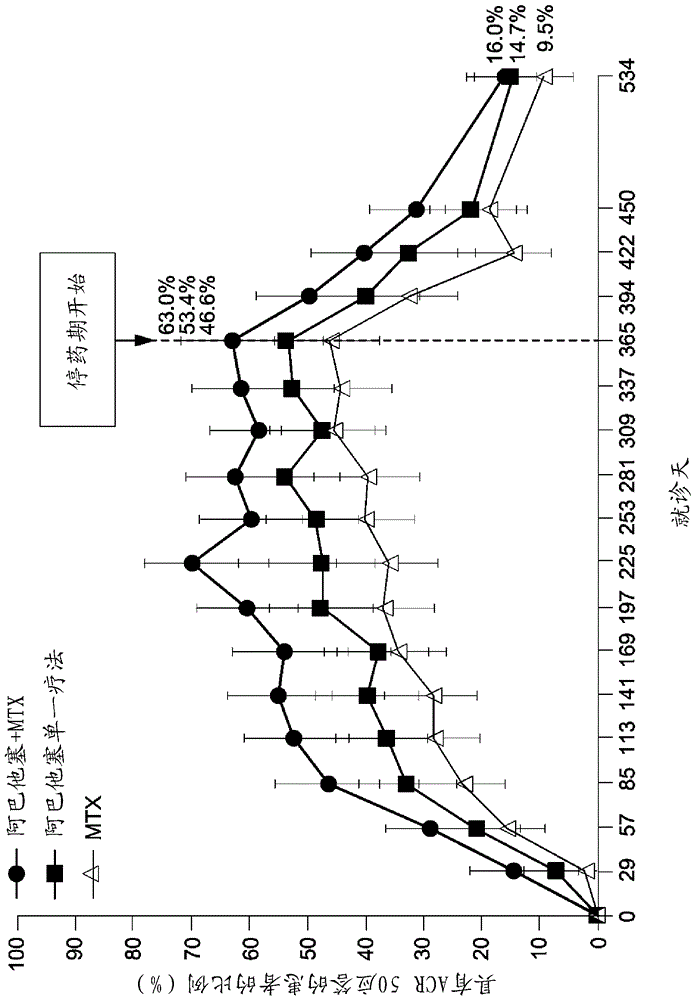
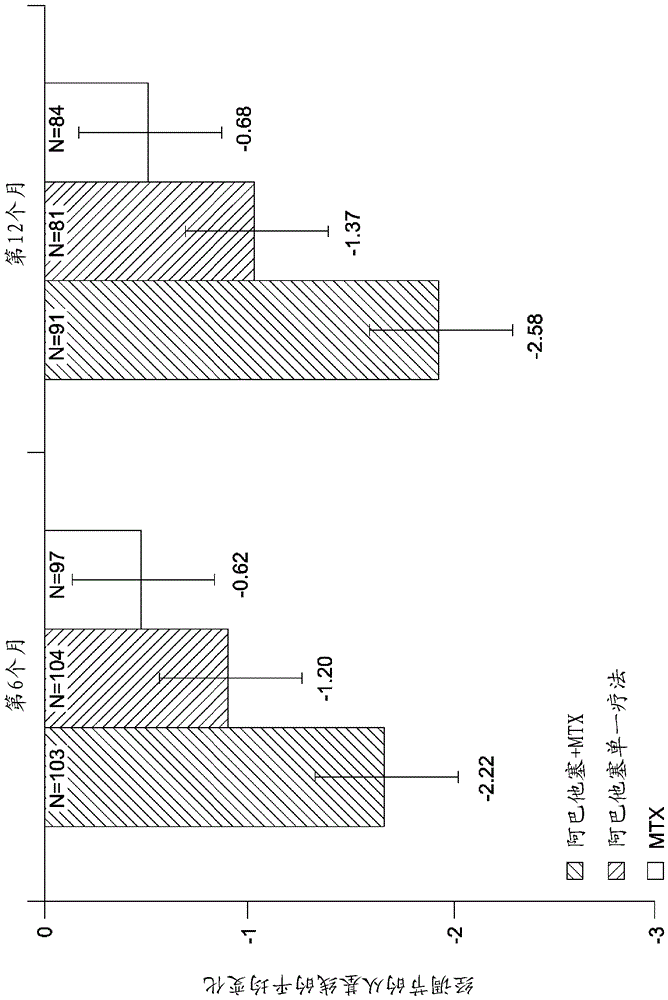
[0171] Evaluating Very Early Rheumatoid Arthritis Therapy (AVERT) is a Phase 3b, randomized, active-controlled 24-month trial with a 12-month double-blind treatment period.
[0172] Research design
[0173] The study design is described graphically in the figure 2 middle.
[0174] include standard
[0175] ●Willing to participate in the study and provided signed informed consent
[0176] Active clinical synovitis in ≥2 joints (including ≥1 facet joint and excluding the distal interphalangeal joint) for ≥8 weeks at Screening
[0177] Onset of persistent symptoms ≤ 2 years prior to screening
[0178]Disease Activity Score 28 (DAS28) C-reactive protein (CRP) ≥ 3.2 at Screening
[0179] ●Anti-cyclic citrullinated peptide-2 positive
[0180] Methotrexate (MTX) naïve or MTX ≤10 mg / kg for ≤4 weeks and dosed less than 1 month prior to screening
[0181] ●Biologically primitive
[0182] Withhold chloroquine, hydroxychloroquine, and sulfasalazine for ≥28 days (if accepted)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com