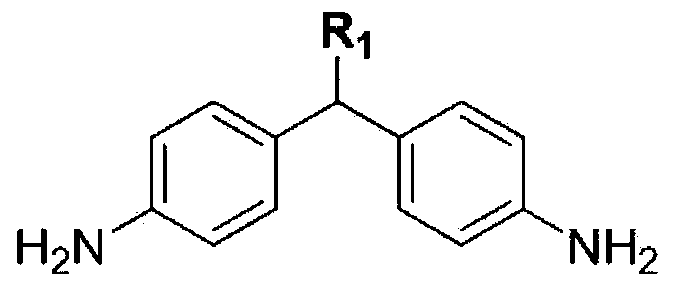
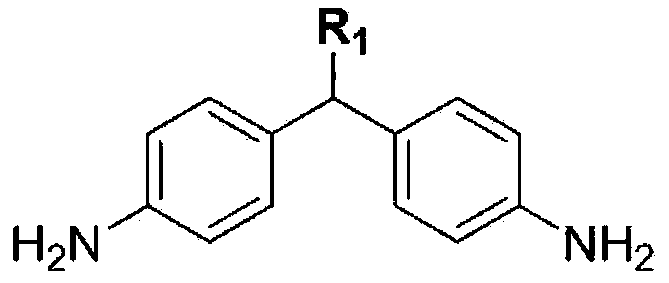
Synthesis of Novel Diamine and Its Liquid Crystal Alignment Agent
A side chain type diamine and vertical alignment technology, which is applied in the field of new diamine synthesis and liquid crystal aligning agent using the same, can solve the problems of low reactivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
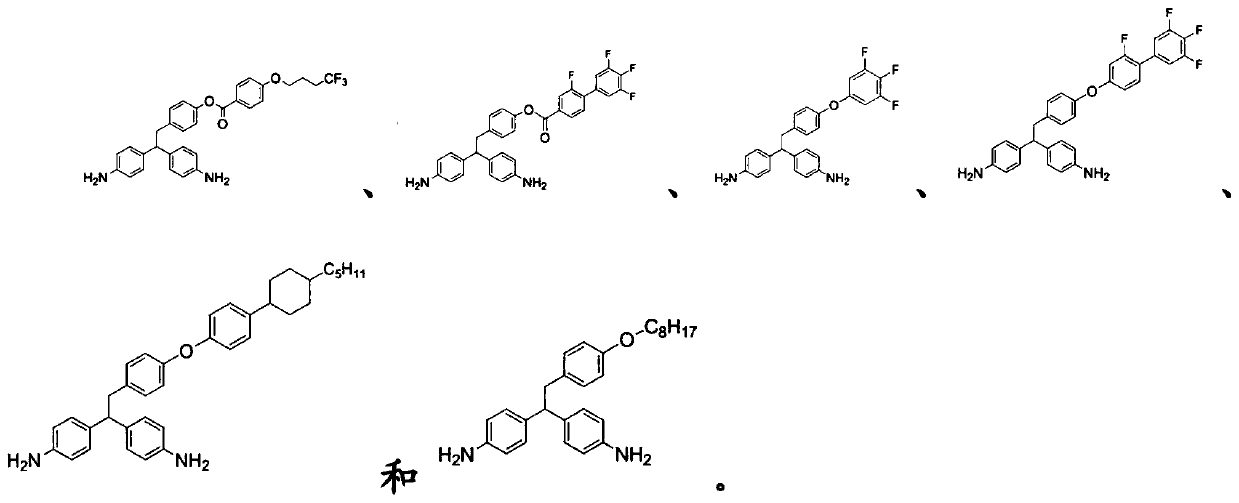
Examples
Synthetic example 1
[0092] (Di-tert-butyl((2-(4-hydroxyphenyl)ethane-1,1-diyl)bis(4,1-phenylene))iminodicarboxylic acid)(di-tert-butyl(( Synthesis of 2-(4-hydroxyphenyl)ethane-1,1-diyl)bis(4,1-phenylene))dicarbamate)
[0093]
[0094] Add 4-(2,2-bis(4-aminophenyl)ethyl)phenol (4-(2,2-bis(4-aminophenyl)ethyl)phenol) (15.0g, 49.3mmol) in the reaction vessel, Further dichloromethane (200 mL) was added for dissolution. Di-tert-butyl dicarbonate (di-tert-butyl dicarbonate) (24.9 ml, 108.4 mmol) was added dropwise to the reaction vessel under an ice bath, followed by stirring at normal temperature for 12 hours. After adding water to the reaction vessel to complete the reaction, extraction was performed with dichloromethane to evaporate all the organic solvents. The resulting mixture was separated by column chromatography (silica gel, hexane / ethyl acetate=1 / 1) to obtain a pale yellow solid (14.9 g, 61%).
[0095] 1 H NMR (300MHz, CDCl 3 )δ7.20(d,4H),7.06(d,4H),6.81(d,2H),6.61(d,2H),6.35(s,2H),4....
Synthetic example 2
[0101] 4-(2,2-Bis(4-aminophenyl)ethyl)phenyl 2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-carboxylate Synthesis of (4-(2,2-bis(4-aminophenyl)ethyl)phenyl 2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-carboxylate)
[0102]
[0103] In a reaction vessel, di-tert-butyl((2-(4-hydroxyphenyl)ethane-1,1-diyl)bis(4,1-phenylene))iminodicarboxylic acid (9g, 17.86 mmol) was dissolved in dichloromethane (200mL), and 2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-carboxylic acid (4.83g, 17.86mmol) was added , DCC (3.68g, 17.86mmol), DMAP (0.22g, 1.79mmol), reacted at room temperature for 12 hours to produce 4-(2,2-bis(4-((tert-butoxycarbonyl)amino)phenyl ) ethyl) phenyl 2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-carboxylate (4-(2,2-bis(4-(( tert-butoxycarbonyl)amino)phenyl)ethyl)phenyl 2,3',4',5'-tetrafluoro-[1,1'-biphenyl]-4-carboxylate) (9g, 67%).
[0104] 4-(2,2-bis(4-((tert-butoxycarbonyl)amino)phenyl)ethyl)phenyl 2,3',4',5'-tetrafluoro-[1, 1'-biphenyl]-4-carboxylate (9g, 11.97mmol) was added to...
Synthetic example 3
[0107] 4,4'-(2-(4-(3,4,5-trifluorophenoxy)phenyl)ethane-1,1-diyl)diphenylamine (4,4'-(2-(4 -Synthesis of (3,4,5-trifluorophenoxy)phenyl)ethane-1,1-diyl)dianiline
[0108]
[0109] In a reaction vessel, di-tert-butyl((2-(4-hydroxyphenyl)ethane-1,1-diyl)bis(4,1-phenylene))iminodicarboxylic acid (5g, 9.9 mmol) was dissolved in DMF (100mL), NaOH (1.6g, 39.6mmol) was added, and the reaction was carried out at room temperature for 1 hour, and then 5-bromo-1,2,3-trifluorobenzene (2.1g, 9.9mmol) After being dissolved in DMF, the reaction was carried out for 5 hours to obtain di-tert-butyl ((2-(4-(3,4,5-trifluorophenoxy)phenyl)ethane-1,1-diyl)bis (4,1-phenylene))iminodicarboxylic acid (di-tert-butyl((2-(4-(3,4,5-trifluorophenoxy)phenyl)ethane-1,1-diyl)bis(4 ,1-phenylene)) dicarbamate) (5.5g, 87%). Di-tert-butyl((2-(4-(3,4,5-trifluorophenoxy)phenyl)ethane-1,1-diyl)bis(4,1-phenylene) at 0°C )) iminodicarboxylic acid (5.5g, 8.66mmol) was added into TFA (30mL) and reacted for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com