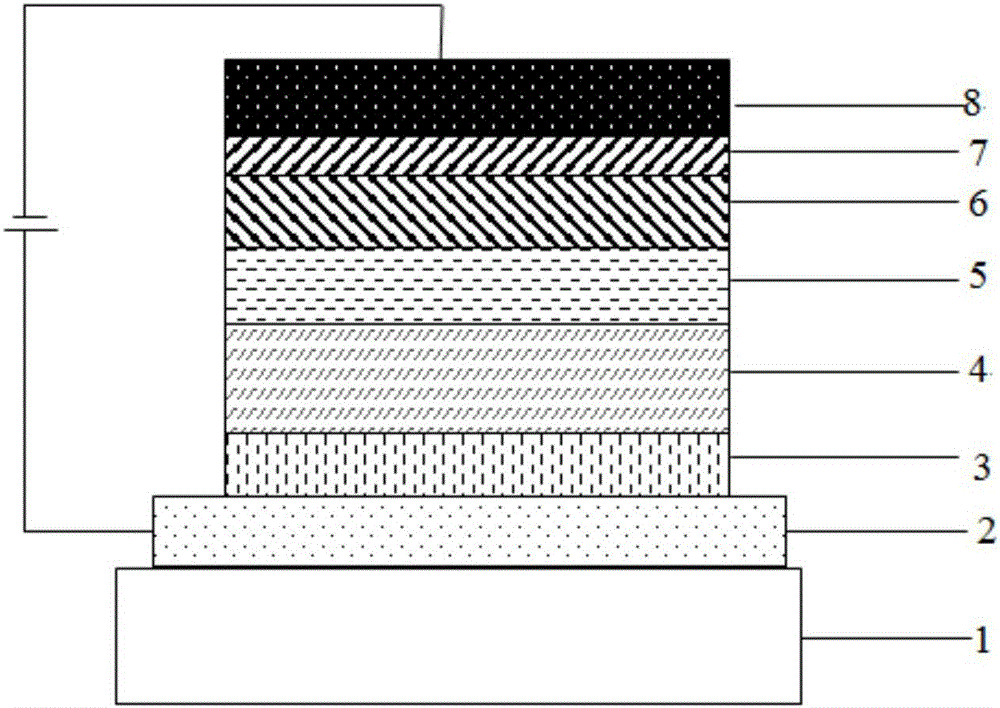
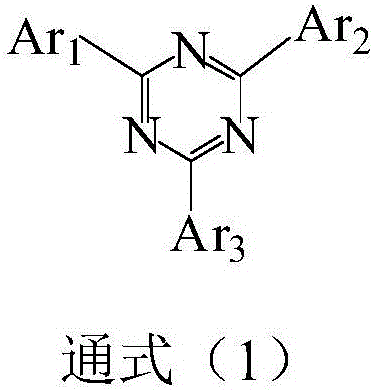
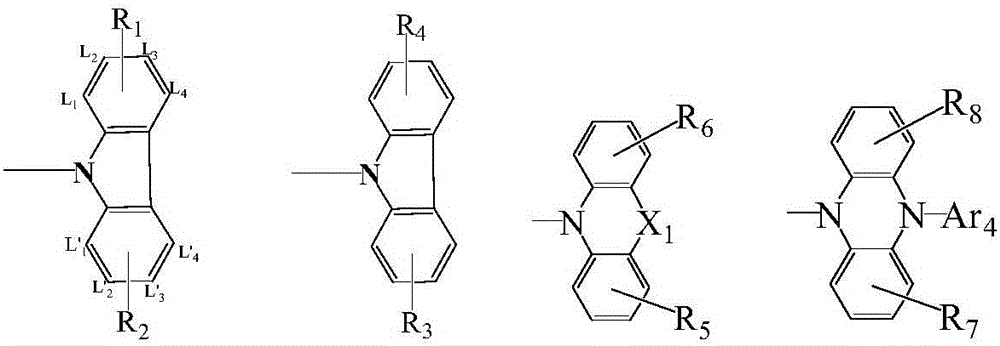
Compound taking triazine as core and application of compound on organic electroluminescent device
A compound and compound structure technology, applied in the fields of electric solid-state devices, electrical components, organic chemistry, etc., can solve the problems of difficult exciton utilization, high fluorescence radiation efficiency, low S1 state radiation transition rate, efficiency roll-off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The synthesis of embodiment 1 compound 1
[0068]
[0069] The concrete synthetic route of this compound is provided now:
[0070]
[0071] In a 250ml four-neck flask, add 0.01mol 2-(4-bromopyridin-2-yl)-4,6-diphenyl-[1,3,5]triazine, 0.015mol12 ,12-Dimethyl-12,14-dihydrobenzofuro[2,3-h]indole[2,1-b]carbazole, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol tri-tert-butylphosphine, 150ml toluene, heating and reflux for 24 hours, sampling point plate, reaction complete, natural cooling, filtration, filtrate rotary evaporation, silica gel column, to obtain the target product with a purity of 99.20% and a yield of 45.39%.
[0072] HPLC-MS: The molecular weight of the material is 681.25, and the measured molecular weight is 681.53.
Embodiment 2
[0073] The synthesis of embodiment 2 compound 12
[0074]
[0075] The concrete synthetic route of this compound is provided now:
[0076]
[0077] In a 250ml four-neck flask, under an atmosphere of nitrogen gas, add 0.01mol 2-(4-bromophenyl)-4-naphthyl-2-yl-6-pyridin-3-yl-[1,3,5 ] Triazine, 0.015molM1, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, reaction complete, natural cooling, filtration, filtrate rotary evaporation, silica gel column, to obtain the target product with a purity of 98.36% and a yield of 52.23%.
[0078] HPLC-MS: The molecular weight of the material is 737.21, and the measured molecular weight is 737.23.
Embodiment 3
[0079] The synthesis of embodiment 3 compound 16
[0080]
[0081] The concrete synthetic route of this compound is provided now:
[0082]
[0083] In a 250ml four-necked flask, add 0.01mol 2-(3-bromophenyll)-4,6-diphenyl-[1,3,5]triazine, 0.015molM2, 0.03mol under nitrogen atmosphere Sodium tert-butoxide, 1 x 10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol tri-tert-butylphosphine, 150ml toluene, heated to reflux for 24 hours, sampling plate, reaction complete, natural cooling, filtration, filtrate rotary evaporation, silica gel column, the target product was obtained with a purity of 98.96% and a yield of 36.98%.
[0084] HPLC-MS: The molecular weight of the material is 736.26, and the measured molecular weight is 736.38.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com