Novel estrogen-related receptor alpha inhibitor and medical application thereof
A technology of estrogen and inhibitors, which is applied in the field of new estrogen-related receptor α inhibitors, and achieves the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
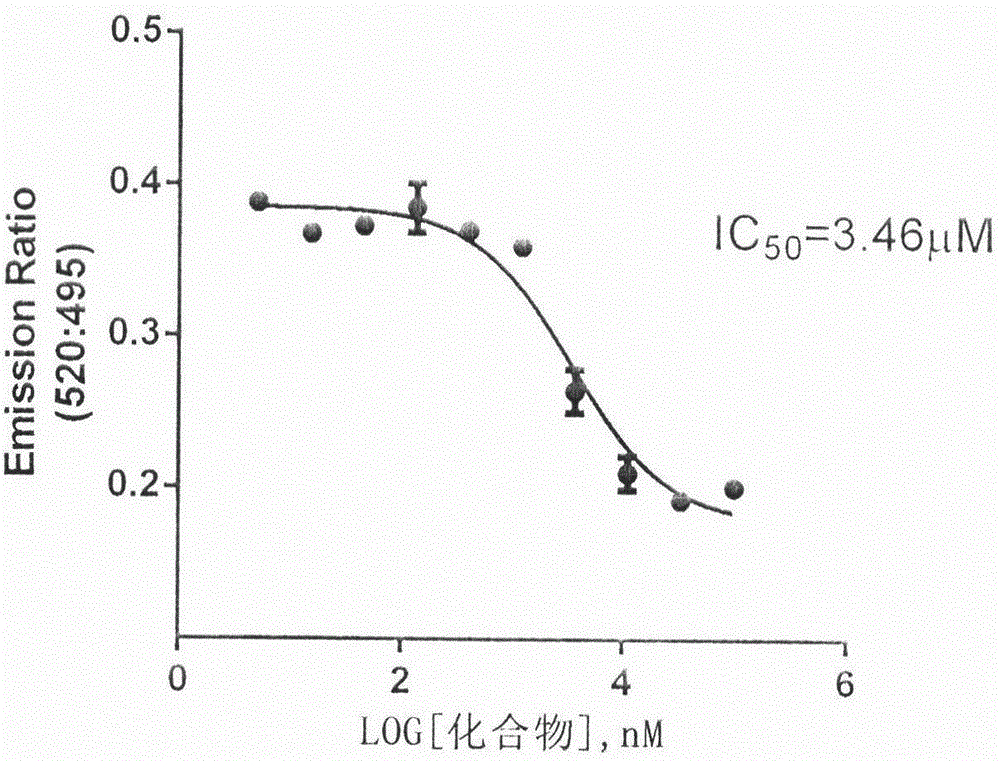
[0025] The time-resolved fluorescence resonance energy transfer detection technology (Time-Resolved Fluoresence Resonance Energy Transfer Assay, TR-FRET Assay) was used to test the competitive binding activity of the compounds of the present invention to ERRα at the molecular level.
[0026] This example illustrates that the compounds involved in the present invention can effectively inhibit the binding of ERRα to co-activator PGC-1α, indicating that the compounds involved in the present invention competitively bind to ERRα (molecular level). TR-FRET is a technique familiar to those skilled in the art.
[0027]FRET (Fluoresence Resonance Energy Transfer) is fluorescence resonance energy transfer, which is based on the energy transfer of two fluorescent groups (donor and acceptor). In the case of two fluorescent groups in close proximity, the interaction between biomacromolecules can be measured with fluorescent labels and energy transfer between the two. When the two groups a...
Embodiment 2
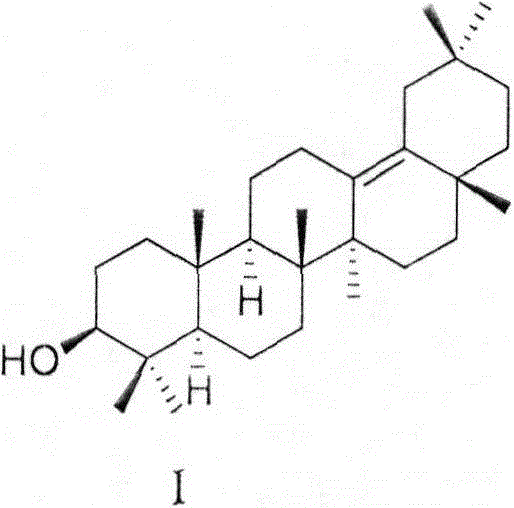
[0043] The preparation of formula I compound
[0044] Step 1, preparation of compound 2
[0045]
[0046] 3β-Hydroxyoleanane-13(18)-ene-28-carboxylic acid (1) (20 g, 0.044 mol) was dissolved in THF (600 mL), and LiAlH was added in portions 4 (7 g, 0.183 mol). Stir at reflux for 1.5 hours. Add methanol and water to quench, extract with ethyl acetate, combine organic layers, wash with saturated brine, and dry over anhydrous sodium sulfate. After spin-drying, a white solid 2 (17 g, yield 88%) was obtained by silica gel column chromatography. IR (film, cm -1 )3374, 2928, 2868, 2359, 1633, 1455, 1384, 1359, 1044, 996, 971; 1 H NMR (300MHz, DMSO-d6) δ3.60 (brs, 2H), 3.23 (dd, J = 5.5, 10.9Hz, 1H), 2.75-2.69 (m, 1H), 2.32 (d, J = 14.1Hz, 1H), 1.93-0.72(m, 22H), 1.18(s, 3H), 0.99(s, 3H), 0.94(s, 3H), 0.86(s, 6H), 0.77(s, 3H), 0.74(s ,3H); 13 C NMR (75MHz, DMSO-d6) δ136.9, 130.9, 77.3, 61.1, 55.4, 50.5, 44.5, 40.9, 39.2, 39.0, 38.9, 37.3, 35.2, 34.9, 33.2, 32.5, 32.2, 29.0, ...
Embodiment 3
[0066] tablet
[0067] The formula I compound (50g) prepared in Example 2, hydroxypropylmethylcellulose E (150g), starch (200g), povidone K30 appropriate amount and magnesium stearate (1g) were mixed, granulated, pressed piece.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com