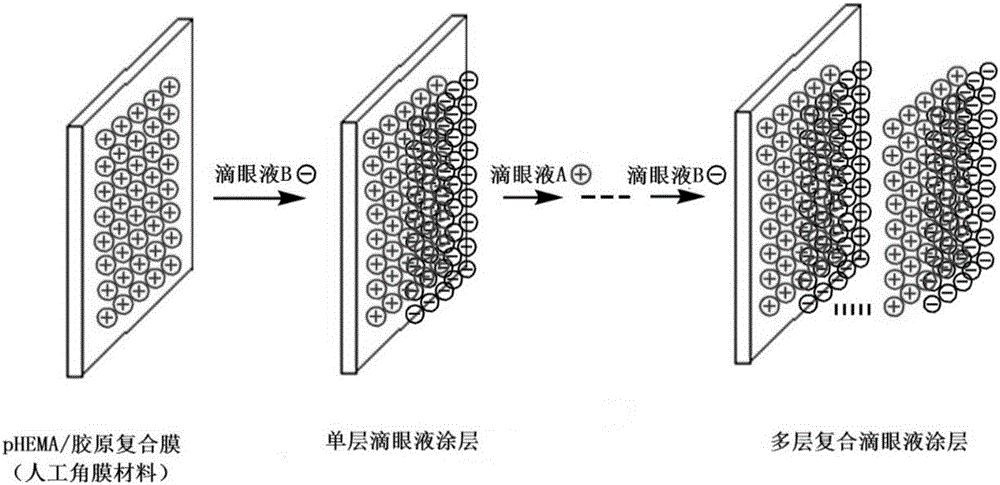
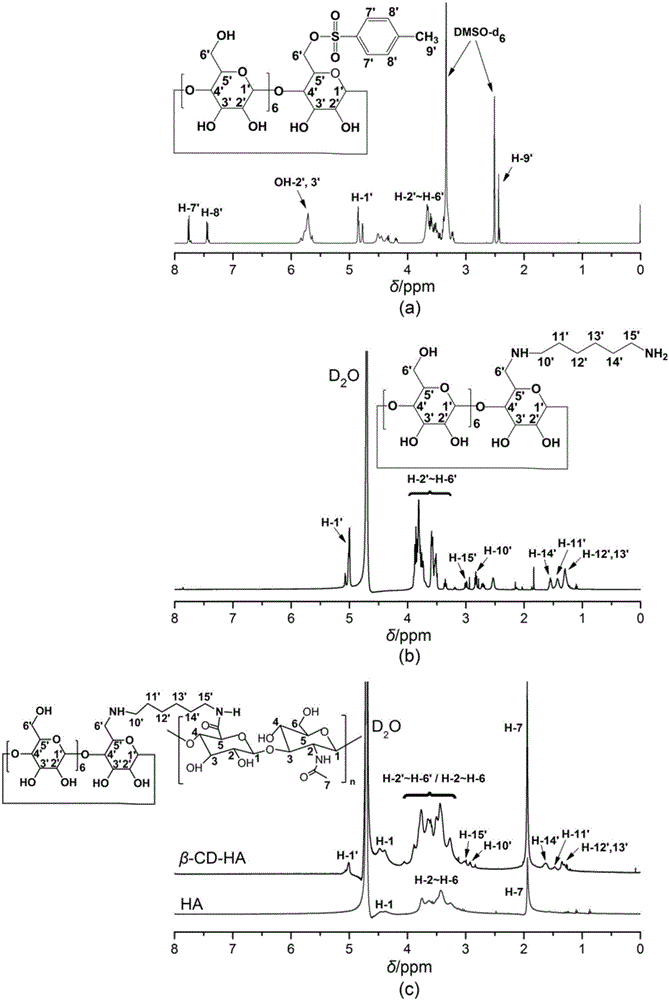
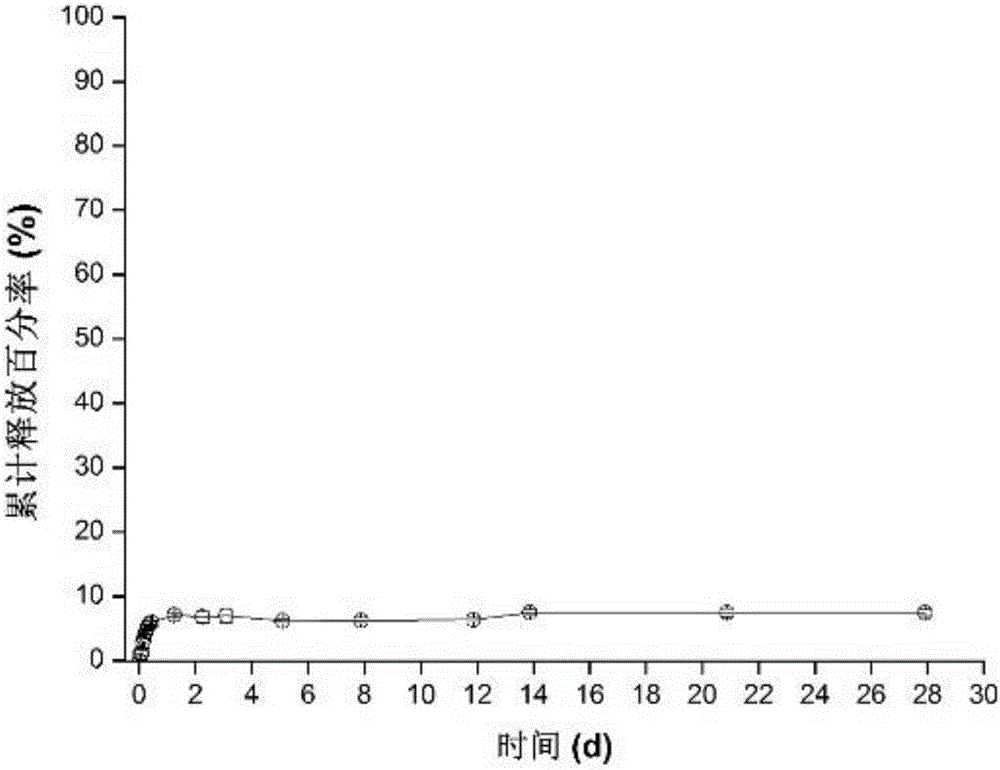
Drug sustained release type composite eye drops and preparation method and application thereof
An eye drop, slow-release technology, applied in the field of biomedical materials, can solve the problems of inability to effectively prevent the rapid migration of small molecule drugs, the inability to realize drugs, weak van der Waals force, etc., to improve drug delivery efficiency and prolong residence time The effect of simple time and preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Preparation of liquid A
[0061] Dissolve 0.3g of collagen in 30mL of 0.1mol / L dilute hydrochloric acid to prepare a collagen solution with a concentration of 0.01g / mL and a pH of 4.0 as liquid A;
[0062] (2) Preparation of liquid B
[0063] ① Weigh 0.1mol β-CD and put it in 1000mL water, add 40mL of 0.33g / mL NaOH dropwise under ice cooling to make the solution clear, then add 0.1mol p-toluenesulfonyl chloride (p -TsCl) in acetonitrile solution, stirred for 5h. Filtrate under reduced pressure to remove unreacted p-TsCl, neutralize the filtrate with 1mol / L hydrochloric acid to neutrality, and then let it stand at 4°C for 24 hours. Acylated β-CD.
[0064] figure 2 (a) for sulfonylated β-CD 1 H NMR spectrum, such as figure 2 As shown in the spectrum line in (a), the proton peaks of sulfonylated β-CD are assigned as follows: 2.42ppm (H-9'), 3.24~3.67ppm (H-2'~H-6'), 4.77ppm ( H-1′), 5.73ppm (OH-2′~3′), 7.76ppm (H-7′), 7.44ppm (H-8′); the results showed that th...
Embodiment 2
[0080] (1) Preparation of liquid A
[0081] Dissolve 1.5g of collagen in 30mL of 0.1mol / L dilute hydrochloric acid to prepare a collagen solution with a concentration of 0.05g / mL and a pH of 5.0 as liquid A;
[0082] (2) Preparation of liquid B
[0083] ① Weigh 0.1mol β-CD and put it in 1000mL water, add 40mL of 0.33g / mL NaOH dropwise under ice bath cooling to make the solution clear, then add 0.05mol p-toluenesulfonyl chloride (p -TsCl) in acetonitrile solution, stirred for 5h. Filtrate under reduced pressure to remove unreacted p-TsCl, neutralize the filtrate with 1mol / L hydrochloric acid to neutrality, and then let it stand at 4°C for 24 hours. Acylated β-CD.
[0084] ②Take 10g of the sulfonylated β-CD prepared in step ① and 20g of hexamethylenediamine, mix and dissolve in 60mL of dimethylformamide, stir and react at 75°C for 4h, add 500mL of cold acetone after cooling, a white precipitate is precipitated, reduce Suction pressure filtration, and then successively dissol...
Embodiment 3
[0094] (1) Preparation of liquid A
[0095] Dissolve 3g of collagen in 30mL of 0.1mol / L dilute hydrochloric acid to prepare a collagen solution with a concentration of 0.1g / mL and a pH of 4.0 as liquid A;
[0096] (2) Preparation of liquid B
[0097] ① Weigh 0.1mol β-CD and place it in 1000mL water, add 40mL of 0.33g / mL NaOH dropwise under ice cooling to make the solution clear, and then add 0.02mol p-toluenesulfonyl chloride (p -TsCl) in acetonitrile solution, stirred for 5h. Filtrate under reduced pressure to remove unreacted p-TsCl, neutralize the filtrate with 1mol / L hydrochloric acid to neutrality, and then let it stand at 4°C for 24 hours. Acylated β-CD.
[0098] ②Take 10g of the sulfonylated β-CD prepared in step ① and 20g of hexamethylenediamine, mix and dissolve in 60mL of dimethylformamide, stir and react at 80°C for 2h, add 500mL of cold acetone after cooling, a white precipitate is precipitated, reduce Suction pressure filtration, and then successively dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com