Neisseria gonorrhoeae-salmonella double-effect vaccine, and preparation method and application thereof
A technology of Neisseria gonorrhoeae and Salmonella, applied in the field of medical microbiology, achieves good safety, high loading efficiency, and high antibody level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] 2. Preparation of Salmonella bacterial shadow
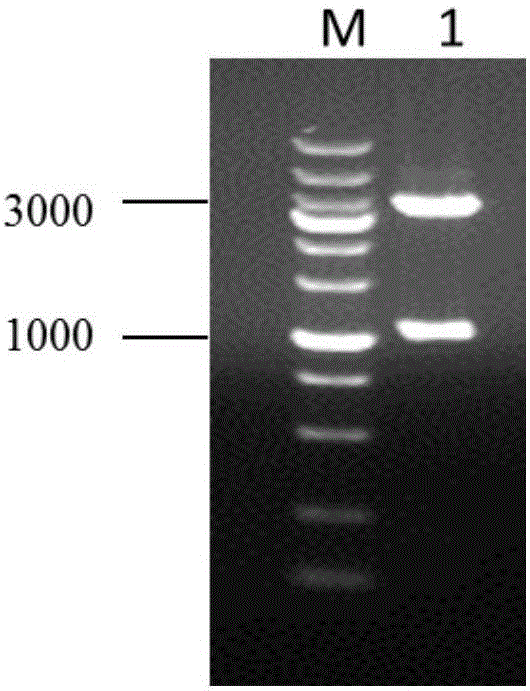
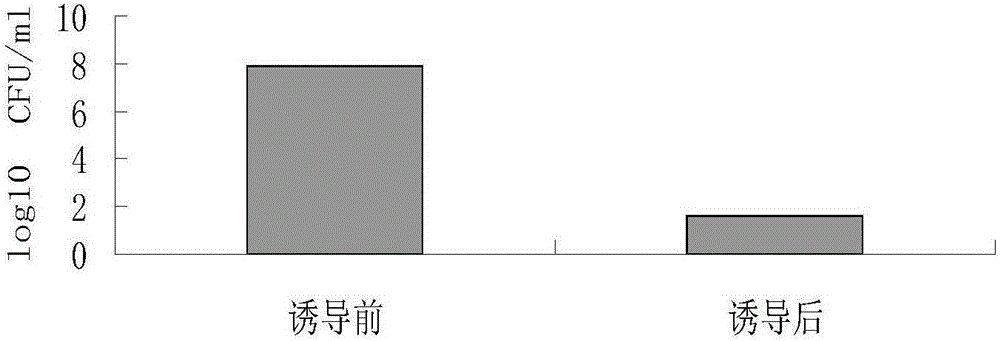
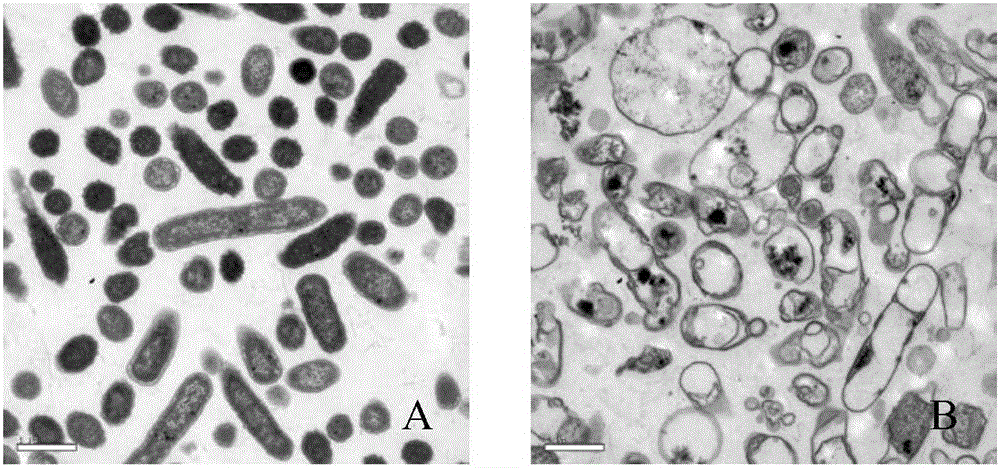
[0034] Insert the phage cleavage gene E into the temperature-controlled expression vector pBV220 to obtain the lytic plasmid p-E, and use p-E as a template. In order to prepare the plasmid pB-E that can induce expression in Salmonella, this part uses the PBBR-MCS2 cloning vector, The lysing cassette amplified by PCR was recombined into the above-mentioned cloning vector and transformed into Salmonella. The recombinant bacteria were inoculated in 5 ml of Kan-resistant LB liquid medium, and cultured with shaking at 200 rpm at 28°C for 8-10 hours. The next day, they were transferred to 2000ml Kan-resistant LB liquid medium at a ratio of 1:500, and cultured at 28°C with shaking at 200rpm. Bacteria were cultured to OD 600 When the value is about 0.3-0.4, rapidly raise the temperature to 42°C to induce expression to prepare Salmonella ghost SE-ghost. Bacterial solutions before and after induction were serially diluted and spr...
Embodiment 2
[0038] In the present embodiment 2, the Neisseria gonorrhoeae-Salmonella double-effect vaccine prepared in the embodiment 1 has been evaluated for immune efficacy, and its specific steps are as follows:
[0039] (1) Evaluation of immune efficacy as gonorrhea vaccine:
[0040] 1) Animal grouping and immunization:
[0041] Thirty 6-week-old SPF BALB / c mice were randomly divided into 3 groups: SE-ghost(PorB) group, pVAX1-PorB group and PBS control group. The mice were deprived of food and water 10 hours before immunization, and 100 μl of 10% sodium bicarbonate solution was orally administered to the mice half an hour before immunization, and water was given to the mice after oral administration for 3 to 4 hours. SE-ghost (PorB) group immunization dose was 1×10 9 CFU, pVAX1-PorB is 100 μg. Vaccine every two weeks, a total of 3 immunizations.
[0042] 2) Detection of humoral immune response level in immunized mice:
[0043] Two weeks after the third immunization, blood was col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com