Method for synthesizing herbicide raw medicine
A synthesis method and herbicide technology, which is applied in the field of synthesis of herbicide technical materials, can solve the problems of inability to industrialize production, large amount of ethanol, and large investment in industrialization, and achieve the effects of simple and reliable production process, high yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
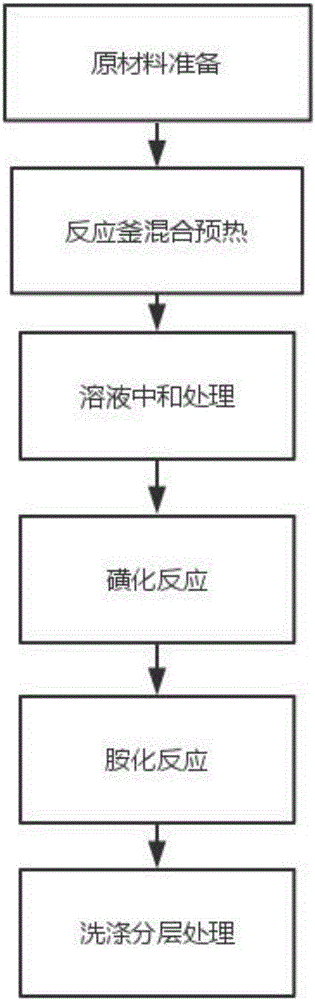
[0020] like figure 1 As shown, the synthetic method of a kind of herbicide former drug of the present embodiment comprises the following processing steps:
[0021] (1) Preparation of raw materials: the raw materials for the production of the herbicide technical drug include the following components (parts by weight): 30 parts of sec-butylamine, 80 parts of 2,6-dinitro-p-tertiary chlorobenzene, 40 parts of NaOH solution, clear water 100 parts, 15 parts of sodium metabisulfite, wherein the purity of sec-butylamine is 99%, and the concentration of NaOH solution is 40%;
[0022] (2) Mixing and preheating of the reaction kettle: add 2,6-dinitro-p-tertiary chlorobenzene and water into the reaction kettle, heat and stir, and the heating temperature is 80°C. After stirring, let stand for a period of 15min, the upper layer waste liquid is separated after standing, and the stirring time is 20min;
[0023] (3) solution neutralization treatment: add NaOH solution to the solution after s...
Embodiment 2
[0027] Example 2: The rest are the same as Example 1, except that the production raw materials in the step (1) include the following components (parts by weight): 25 parts of sec-butylamine, 2,6-dinitro-p-tertyl 70 parts of chlorobenzene, 35 parts of NaOH solution, 90 parts of clear water, and 12 parts of sodium metabisulfite, wherein the purity of sec-butylamine is 98%, the concentration of NaOH solution is 38%, the stirring time in the step (2) is 18min, heating The temperature is 70°C, the standing time is 12min, the heating temperature in the step (4) is 80°C, the stirring time is 20min, and the standing time is 25min. The addition time is 1.2h, the reaction temperature is 45°C, the reaction time is 1.2h, and the heating temperature in the step (6) is 95°C.
Embodiment 3
[0028] Example 3: the rest are the same as Example 1, except that the production raw materials in the step (1) include the following components (parts by weight): 30 parts of sec-butylamine, 2,6-dinitro-p-tertyl 80 parts of chlorobenzene, 40 parts of NaOH solution, 100 parts of clear water, and 15 parts of sodium metabisulfite, wherein the purity of sec-butylamine is 99%, the concentration of NaOH solution is 40%, the stirring time in the step (2) is 20min, heating The temperature is 80°C, the standing time is 15min, the heating temperature in the step (4) is 90°C, the stirring time is 25min, and the standing time is 30min. The addition time is 1.2h, the reaction temperature is 50°C, the reaction time is 1.5h, and the heating temperature in the step (6) is 100°C.
[0029] After the above process steps, take out the herbicide technical sample, and obtain the following data:
[0030] serial number purity yield Example 1 95.6% 92% Example 2 96.4% 92.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com