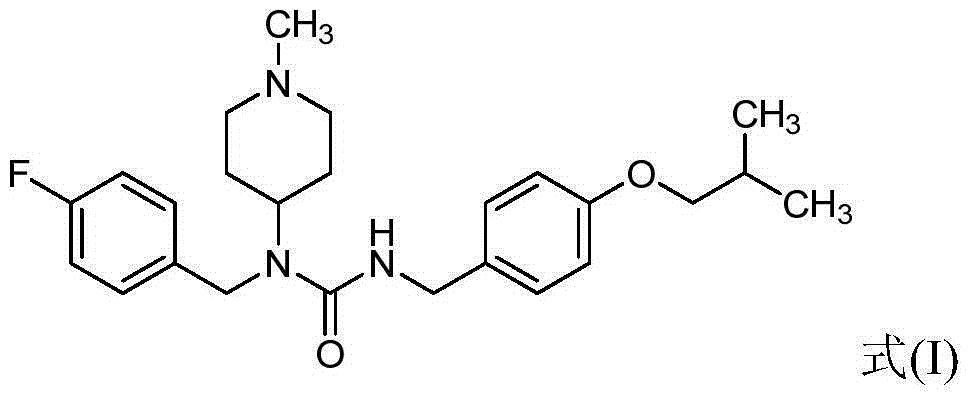
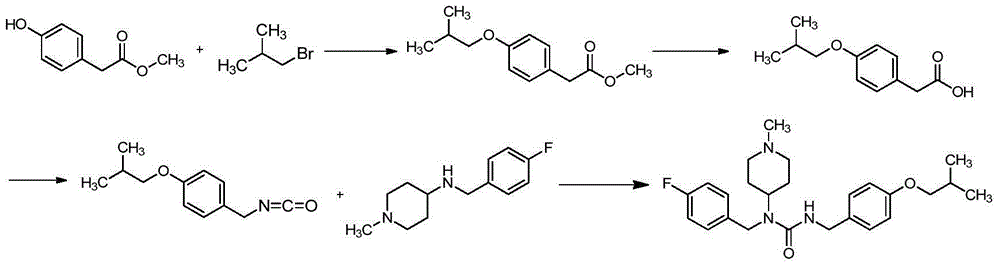
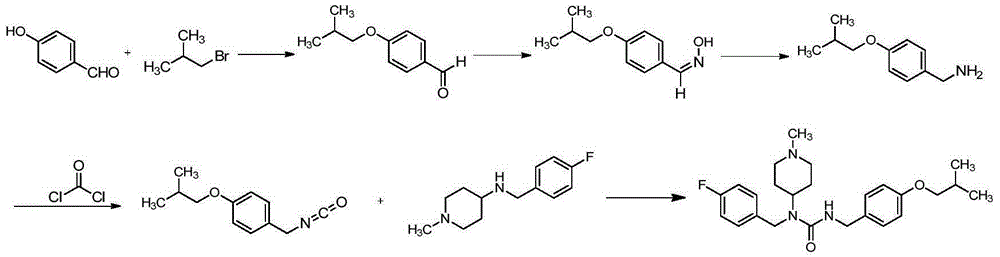
Preparation method for pimavanserin
A technology of pimovanserin and equation, applied in the direction of organic chemistry, etc., can solve the problems of many steps and complex synthesis conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Add dichloromethane (5.0g) and 4-isobutoxybenzylamine (1.0g) into the reaction tube, cool down to 0-5°C, add N,N'-carbonyldiimidazole (1.0g) , kept at 0-5°C for half an hour, added N-(4-fluorobenzyl)-1-methylpiperidin-4-amine (1.0 g), stirred at 0-5°C for 6 hours, raised the temperature to 22°C, kept Overnight (12h), water (3×10g) was added for washing, and the organic phase was taken and concentrated to obtain a viscous oil, which was separated by column chromatography as a white powdery solid (1.17g, yield 61.0%).
[0037] 1 H NMR: (400MHz,d-DMSO)δ:7.27-7.23(m,2H),7.13-7.09(m,2H),6.88-6.85(t,1H),6.85-6.83(m,2H),4.41( d,J=5.6Hz,2H),3.93-3.88(m,1H),3.71(d,J=5.6Hz,2H),2.70(d,J=11.2Hz,2H),2.31(s,1H), 2.10(s,3H),2.03-1.96(m,1H),1.90-1.85(m,2H),1.56-1.50(m,2H),1.44-1.41(m,2H),0.98(s,3H), 0.96(s,3H);
[0038] 13 C NMR: (100MHz, d-DMSO) δ: 162.5, 160.1, 158.0, 157.9, 137.8, 137.6, 137.5, 133.6, 129.4, 128.9, 128.8, 128.7, 128.6, 125.8, 115.3, 115.1, 114.5, 74....
Embodiment 2
[0040] Example 2: Dissolve 4-isobutoxybenzylamine (100.0g) in dichloromethane (500ml), cool down to 0-5°C, slowly add N,N'-carbonyldiimidazole (100.0g), and Keep at ~5°C for half an hour, add N-(4-fluorobenzyl)-1-methylpiperidin-4-amine (100.0g), slowly raise the temperature to 18~22°C, keep stirring for 12 hours, add water (3 × 500g) washed the organic phase three times, evaporated the organic phase to remove the solvent under reduced pressure, added isopropyl acetate (600g), stirred and crystallized, filtered, and the filter cake was dried to obtain the target product (white powdery solid 163.0g, 85.0%).
Embodiment 3
[0041]Example 3: Dissolve triphosgene (14.8g) in dichloromethane (200ml), lower the temperature to -5 to 5°C, and slowly add 4-isobutoxybenzylamine (17.9g) and triethylamine (30.3 g), continue to keep for 1 hour after adding, slowly add N-(4-fluorobenzyl)-1-methylpiperidin-4-amine (22.0g) dropwise, slowly heat up to 15-25°C after adding, keep stirring After 5 hours, use water (3 * 200g) to wash the organic phase three times, the organic phase is decompressed to remove the solvent, isopropyl acetate (110g) is added, stirred and crystallized, filtered, and the filter cake is dried to obtain the target product (white powder Solids 34.5 g, 81.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com