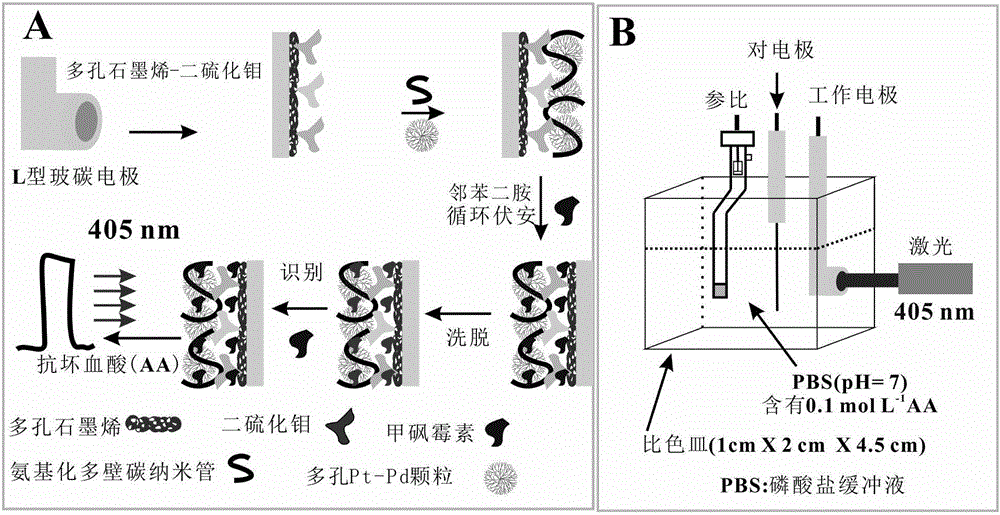
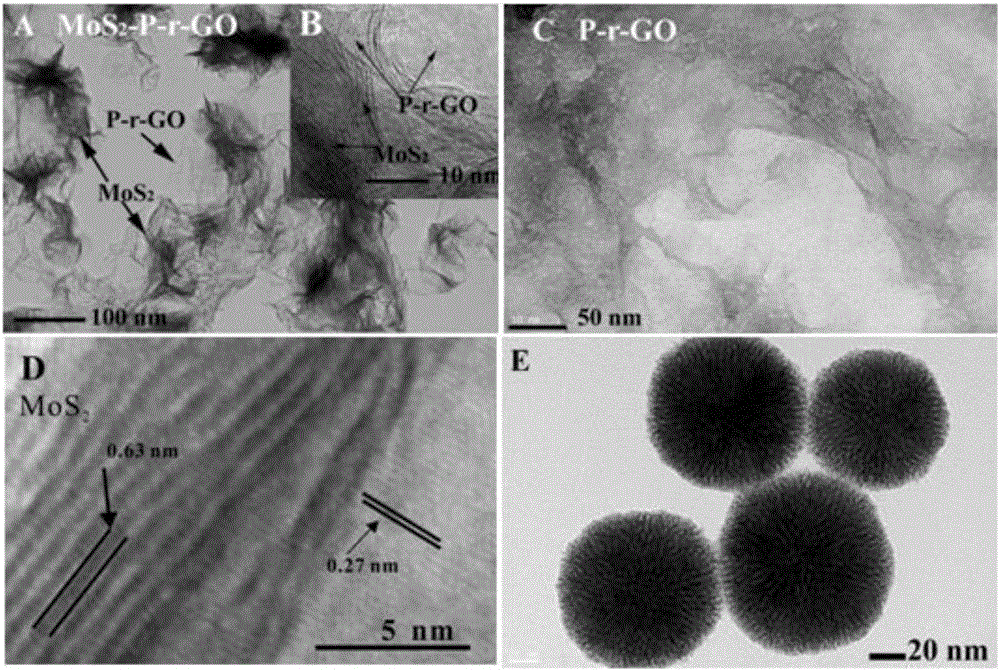
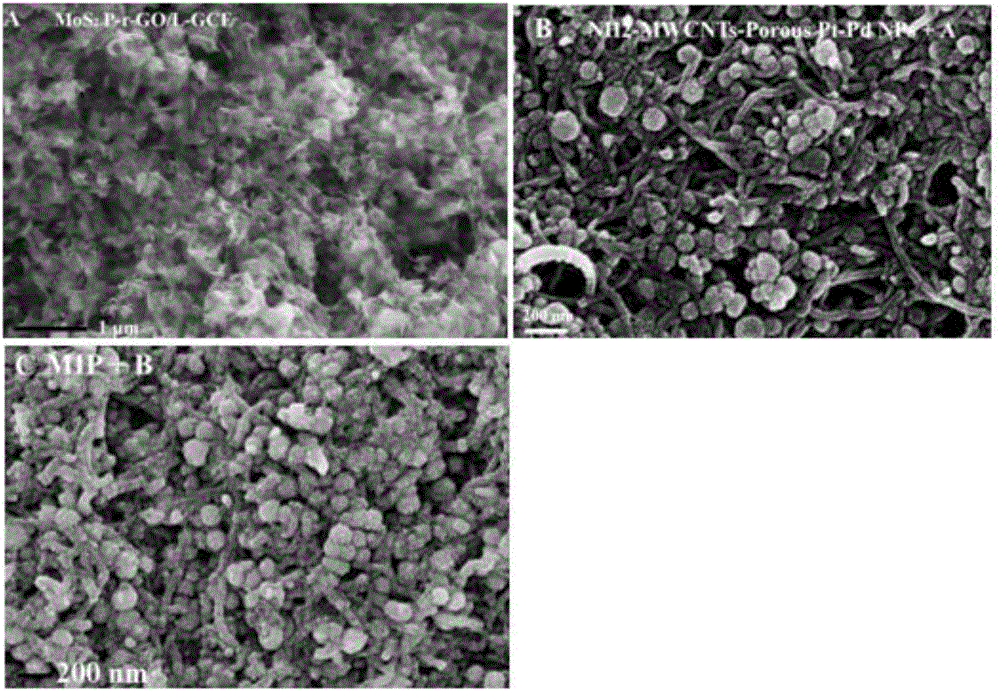
Thiamphenicol molecularly imprinted electrochemical sensor and preparation method and application thereof
A thiamphenicol, molecular imprinting technology, applied in the direction of material electrochemical variables, scientific instruments, instruments, etc., can solve problems such as human health effects, and achieve the effect of broadening detection channels, improving sensitivity, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of porous Pt-Pd NPs: 5.0 mL hexadecylpyridine (HDPC) (4.0 mg mL -1 ), 0.40mLNa 2 PdCl 4 (10mmol L -1 ) and 0.40mL H 2 PtCl 6 (10mmol L -1 ) into a round bottom flask to form a uniform dispersion; then quickly add 0.60mL of freshly prepared AA solution to the above solution, shake gently to make it evenly dispersed, and place the round bottom flask Reaction in an oil bath at 85°C for 3 h; then, the obtained sol was centrifuged and washed with water several times to obtain dendritic Pt-Pd NPs;
[0043] (2) Preparation of porous graphene oxide: under magnetic stirring, 0.5g KMnO 4 Add 100mL 0.5mg mL - 1 GO dispersion and allowed to react for 12 h. Then, 30mL HCl (36%, wt%) and 30mL H 2 o 2 (30%, wt%) was added to the above reaction solution, and the reaction was continued for 3h. After the reaction, the obtained product was centrifuged, washed with water, and dried in a vacuum dryer at 60°C; a porous graphene oxide dispersion was prepared;
[0...
Embodiment 2
[0048] (1) Preparation of porous Pt-Pd NPs: Hexadecylpyridine (HDPC, 10molL -1 ), Na 2 PdCl 4 (10molL -1 ) and H 2 PtCl 6 (10molL -1 ) into a round bottom flask according to the volume ratio of 3:1:1 to form a uniform dispersion; then the freshly prepared ascorbic acid (AA) solution is quickly added to the above solution, the volume ratio of AA solution to HDPC is 1:25, shake gently to make it evenly dispersed, and place the round-bottomed flask in an 80°C oil bath for 2.5h; then, centrifuge the obtained sol and wash it with water several times to obtain dendritic Pt-Pd NPs;
[0049] (2) Preparation of porous graphene oxide:
[0050] Under magnetic stirring, the KMnO 4 Added to the GO dispersion and allowed to react for 6h, wherein, KMnO 4 The mass ratio to GO dispersion is 8:1; then, HCl (36%, wt%) and H 2 o 2 (30%, wt%) was added in the above-mentioned reaction solution, continued reaction 3h, wherein GO dispersion liquid and HCl and H 2 o 2 The volume ratio of ...
Embodiment 3
[0055] (1) Preparation of porous Pt-Pd NPs: Hexadecylpyridine (HDPC, 10molL -1 ), Na 2 PdCl 4 (10molL -1 ) and H 2 PtCl 6 (10molL -1 ) into a round bottom flask according to the volume ratio of 8:1:1 to form a uniform dispersion; then the freshly prepared ascorbic acid (AA) solution is quickly added to the above solution, the volume ratio of AA solution to HDPC is 1:5, shake gently to make it evenly dispersed, and place the round bottom flask in a 90°C oil bath for 3.5h; then, centrifuge the obtained sol and wash it with water several times to obtain dendritic Pt-Pd NPs;
[0056] (2) Preparation of porous graphene oxide:
[0057] Under magnetic stirring, the KMnO 4 Added to the GO dispersion and allowed to react for 12h, wherein, KMnO 4 The mass ratio to GO dispersion is 15:1; then, HCl (36%, wt%) and H 2 o 2 (30%, wt%) was added in the above-mentioned reaction solution, continued reaction 3h, wherein GO dispersion liquid and HCl and H 2 o 2 The volume ratio of ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com