Measurement method for pharmacokinetic parameters based on constant-speed intravenous infusion
A technology of pharmacokinetics and measurement methods, applied in the field of pharmacokinetics, which can solve the problems of low blood drug concentration, large error, and underestimation of the calculation results of the apparent volume of distribution of the drug in the phase of elimination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
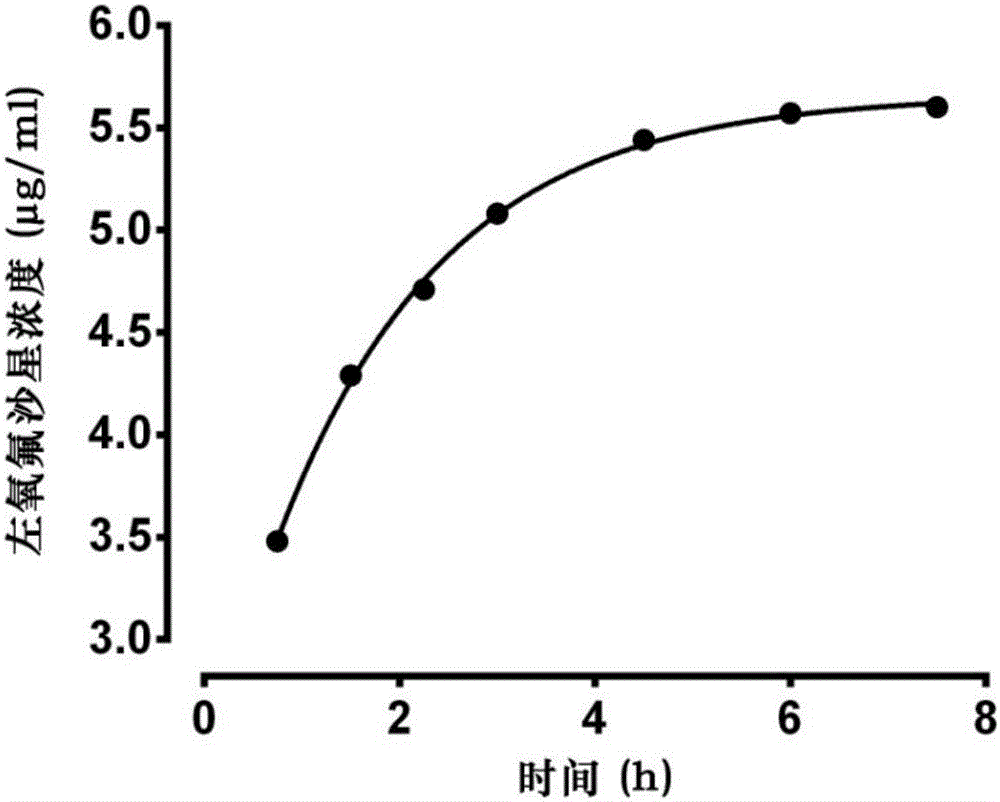
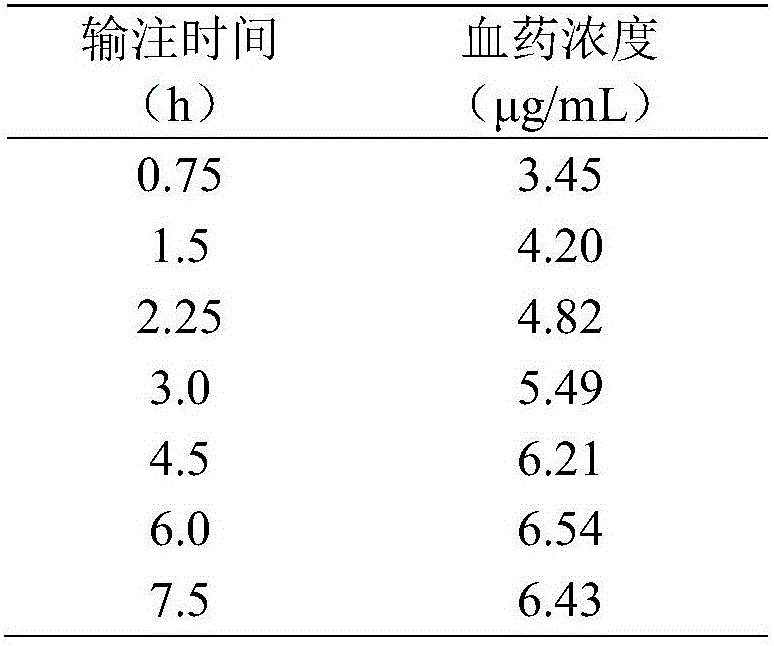
[0081] Embodiment 1: Determination of its main pharmacokinetic parameters by slow and constant intravenous infusion of levofloxacin
[0082] Male SD rats, weighing 360 g, were provided by the Experimental Animal Center of Xi'an Jiaotong University. Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate, the neck, head and back were depilated in a small area, the skin of the neck was cut, the jugular vein and carotid artery were separated, respectively intubated and fixed. Suture the wound and wear a vest. The arteriovenous cannula is passed subcutaneously to the back incision, and the venous cannula is connected to the infusion pump through the spring tube on the back of the vest. The arterial tube is adhered to the spring tube to facilitate blood collection. The upper end of the spring tube is fixed above the mouse cage to ensure that the rats can move freely and cannot grasp and bite the intubation tube. Use the RM-1200 intravenous microinjection pump t...
Embodiment 2
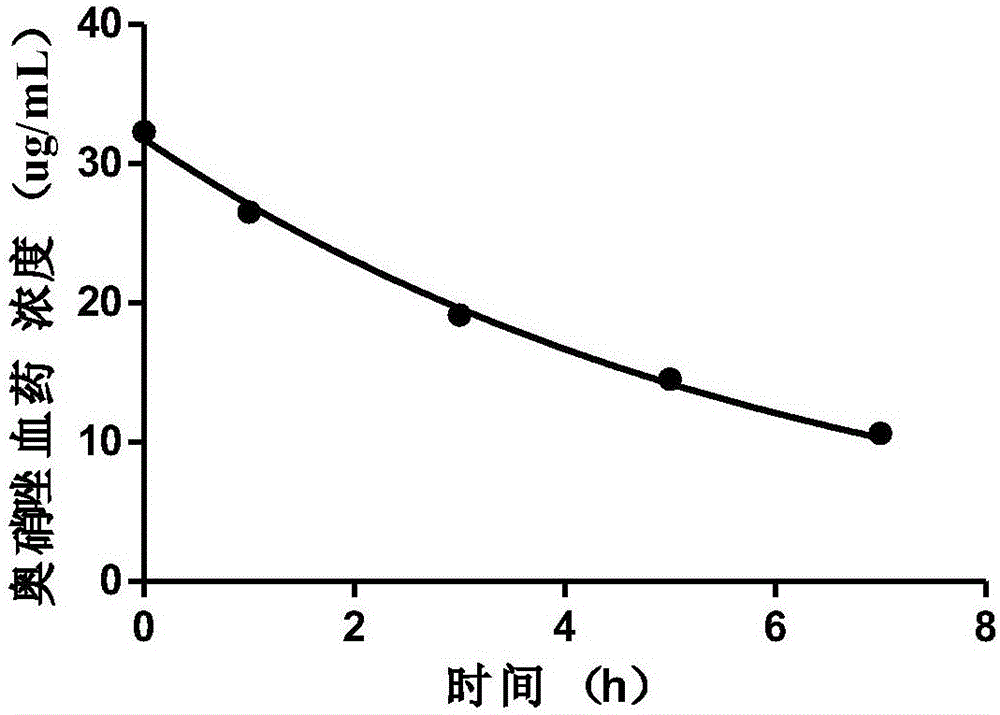
[0102] Embodiment 2: Determination of its main pharmacokinetic parameters after slow and constant intravenous infusion of ornidazole
[0103] Male SD rats, weighing 350 g, were provided by the Experimental Animal Center of Xi'an Jiaotong University. Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate, the neck, head and back were depilated in a small area, the skin of the neck was cut, the jugular vein and carotid artery were separated, respectively intubated and fixed. Suture the wound and wear a vest. The arteriovenous cannula is passed subcutaneously to the back incision, and the venous cannula is connected to the infusion pump through the spring tube on the back of the vest. The arterial tube is adhered to the spring tube to facilitate blood collection. The upper end of the spring tube is fixed above the mouse cage to ensure that the rats can move freely and cannot grasp and bite the intubation tube. Ornidazole and sodium chloride injection (specif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com