Synthesis method for industrial production of octreotide
A synthesis method and octreotide technology, applied in the field of synthesis of octreotide industrial production, can solve the problems of low purity of octreotide finished product, unsuitable synthesis process for large-scale industrial production, low yield, etc., so as to reduce the number of subsequent purifications and avoid reaction time. long, yield-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
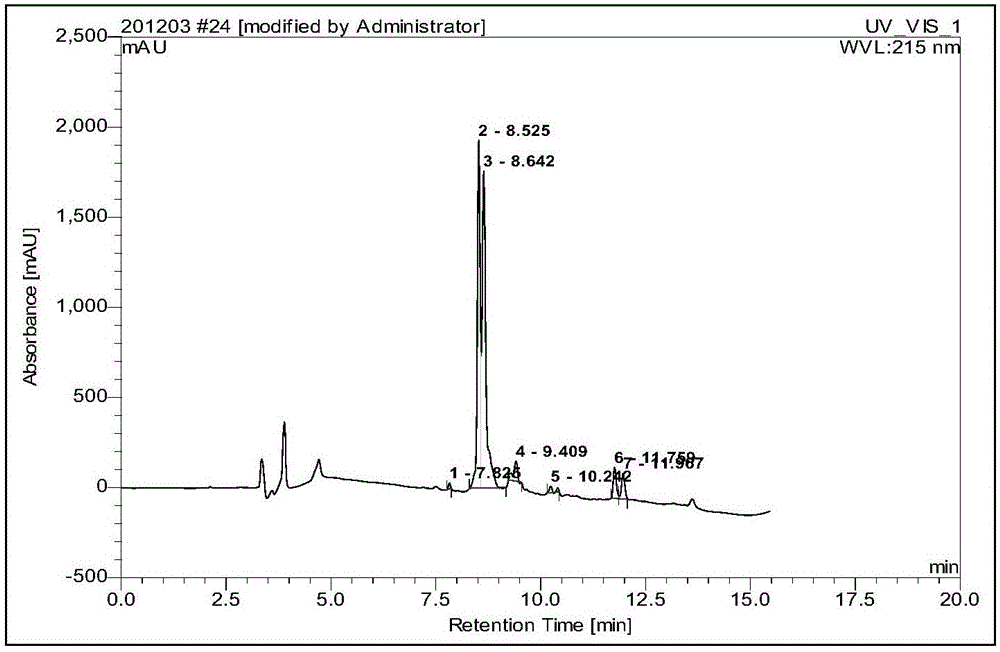
Embodiment 1
[0054] Embodiment 1: A kind of synthetic method of industrialized production of octreotide
[0055] Budget produces 5mmol octreotide, and the synthetic method of octreotide comprises the following steps successively:
[0056] (1) Weigh 8.33g of AM Resin with a substitution degree of 0.60mmol / g and place it in a solid-phase reactor;
[0057] (2) Add 30ml of DCM / DMF mixed solvent to swell the resin for 30 minutes, wherein the volume ratio of DCM to DMF in the DCM / DMF mixed solvent is 1:1~3:1;
[0058] (3) Add 30ml of DMF to wash 6 times, remove the washing solution, weigh 6.6g of Fmoc-Thr-x, 2.025g of HOBT, put into the reaction column, add an appropriate amount of DMF to blow nitrogen, make it stir evenly, and then drop 2.32ml of DIC, reacted for 2~3h, the ninhydrin test showed colorless, if the color developed, repeated re-dosing until the reaction was colorless, and Fmoc-Thr-x-AM Resin was obtained;
[0059] (4) Take out the reaction solution, wash the resin twice with DMF,...
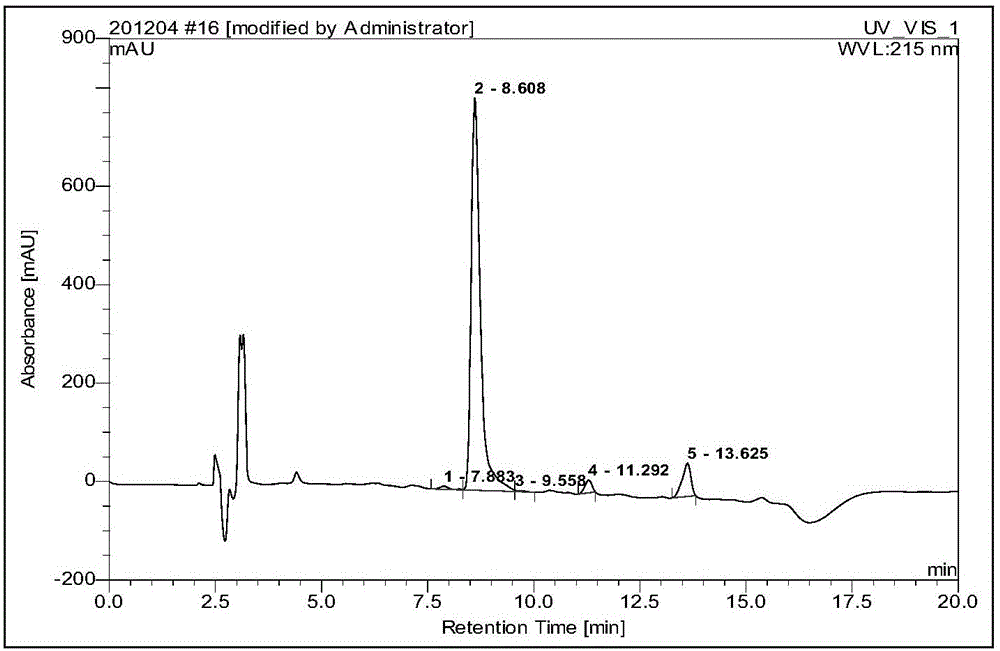
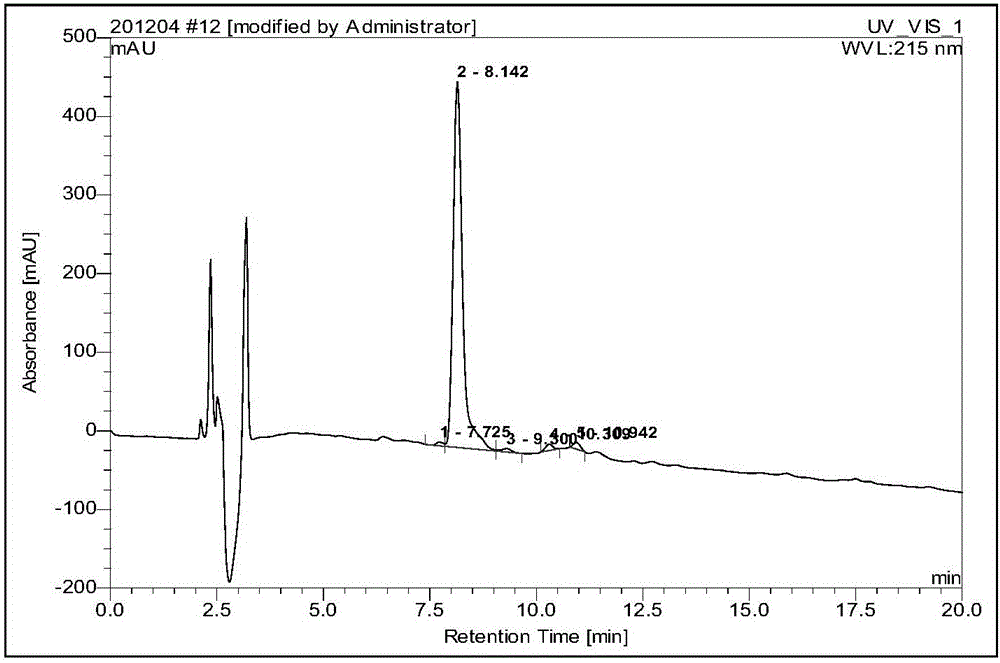
Embodiment 2
[0070] Embodiment 2: A kind of synthetic method of industrialized production of octreotide
[0071] Budget produces 200mmol octreotide, and the synthetic method of octreotide comprises the following steps successively:
[0072] (1) Weigh 350.8g of AM Resin with a substitution degree of 0.57mmol / g and place it in a solid-phase reactor;
[0073] (2) Add 1200ml of DCM / DMF mixed solvent to swell the resin for 30 minutes, wherein the volume ratio of DCM to DMF in the DCM / DMF mixed solvent is 1:1~3:1;
[0074] (3) Take out the swelling solution and add 1200ml of DMF to wash 6 times, take out the washing solution, weigh 265g of Fmoc-Thr-x and 81g of HOBT, put them into the reaction column, add an appropriate amount of DMF and blow nitrogen, make it stir evenly, and then Add 93ml of DIC dropwise, react for 2~3h, the ninhydrin test shows colorless, if the color develops, repeat re-dosing until the reaction is colorless, and get Fmoc-Thr-x-AM Resin;
[0075] (4) Take out the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com