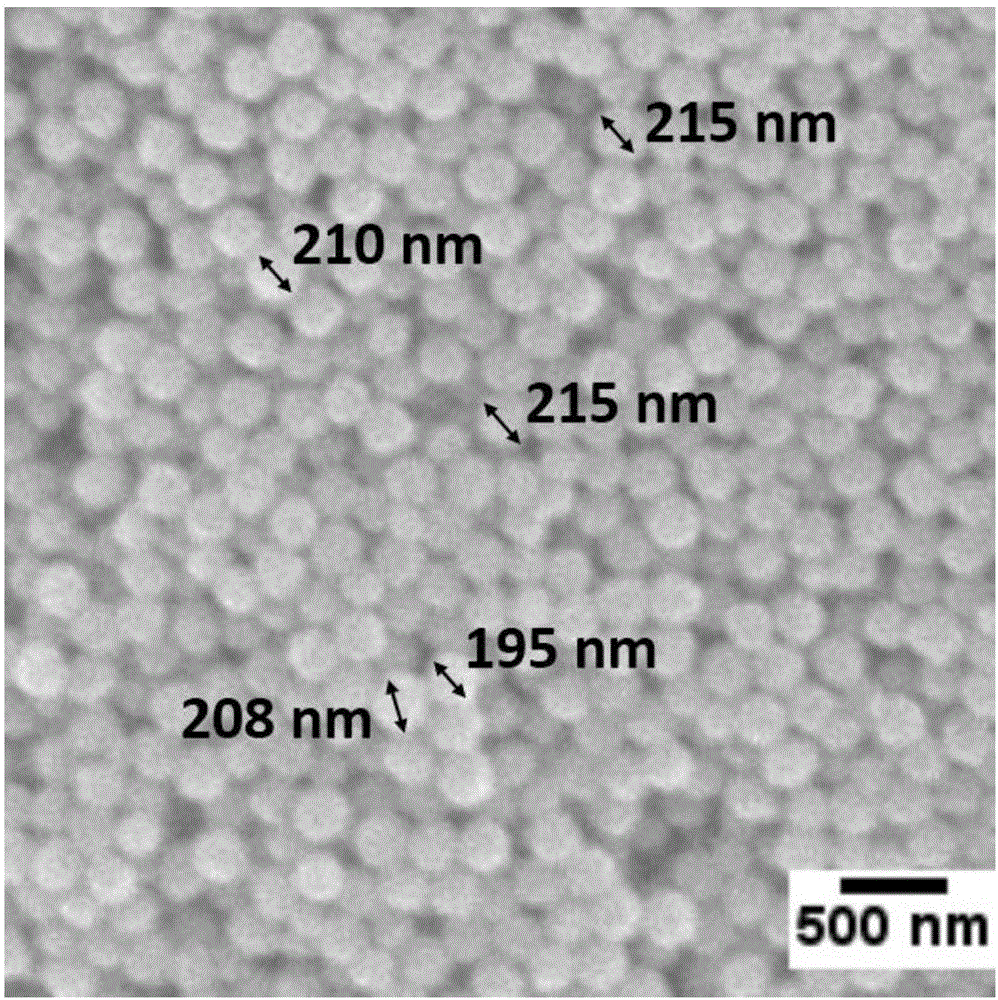
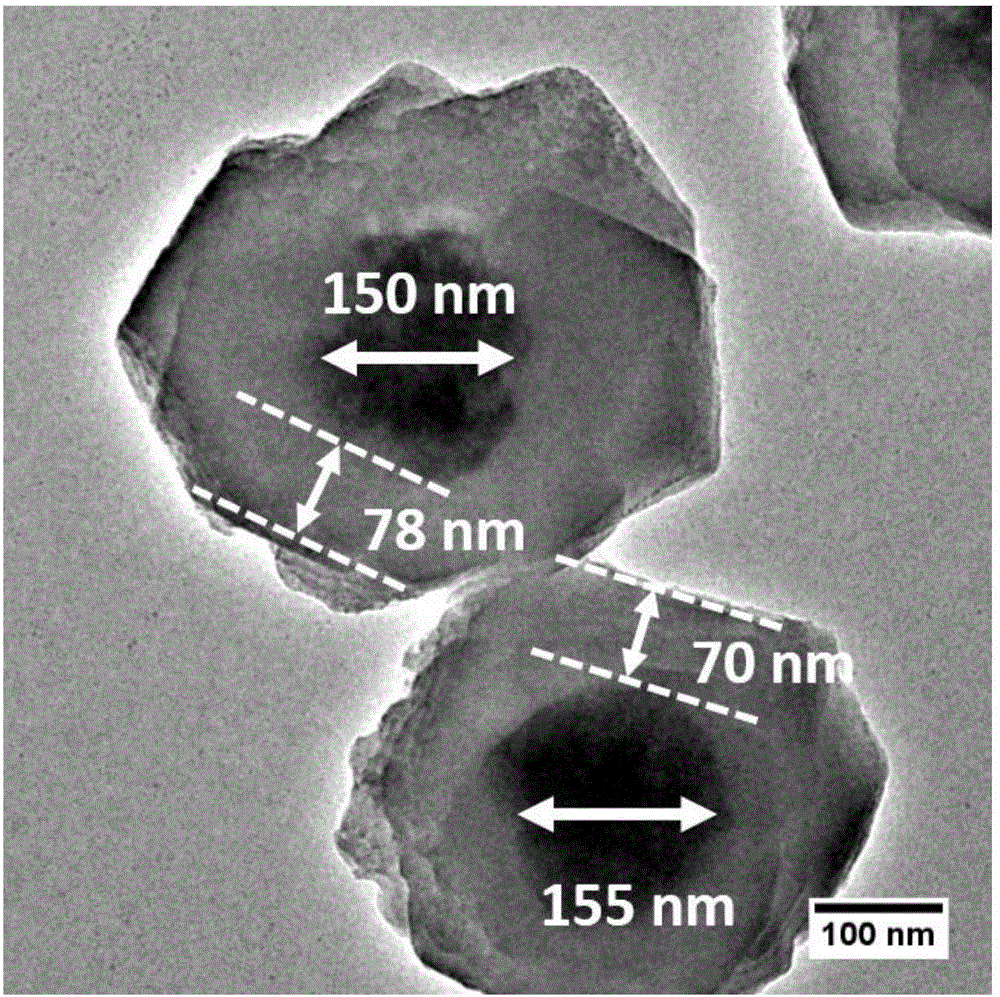
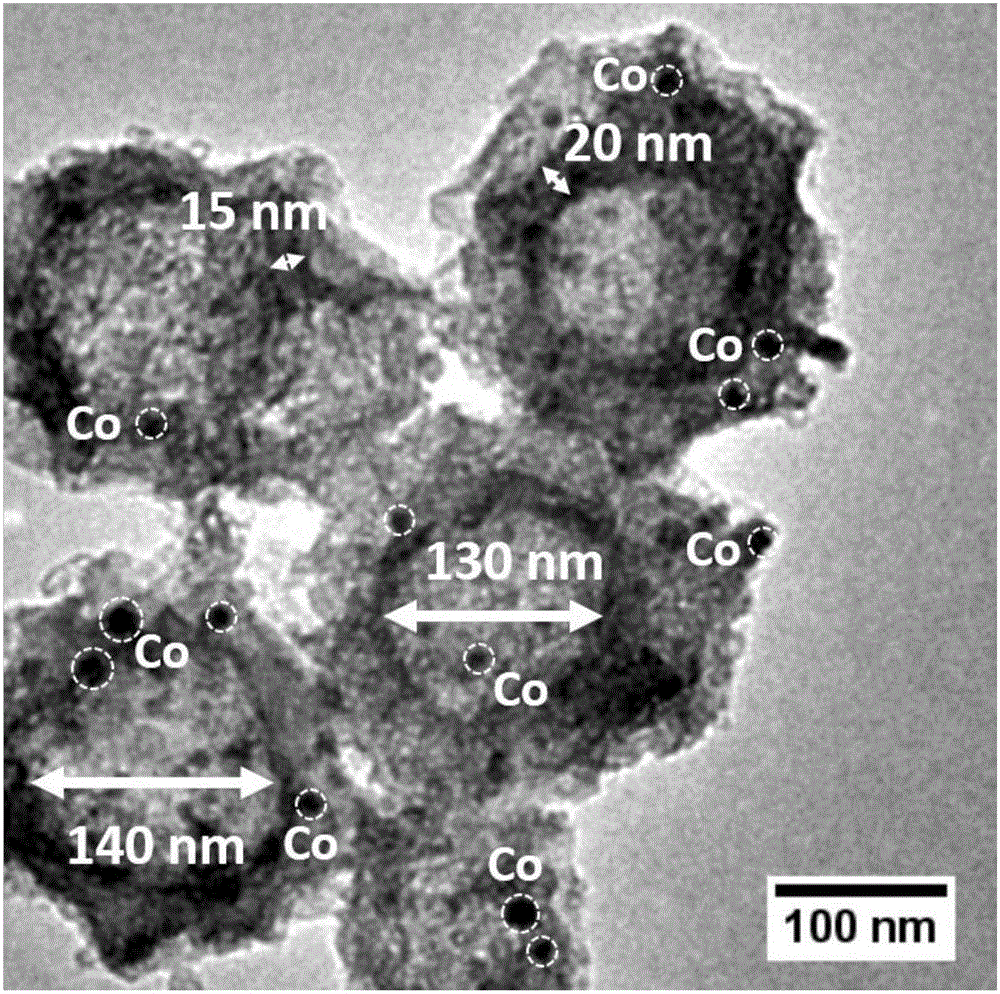
Preparation method of monodisperse cobalt-nitrogen co-doped hollow carbon nano-particles
A carbon nanoparticle and co-doping technology, applied in nanocarbon and other directions, can solve the problems of small specific surface area of carbon materials and achieve the effect of an environmentally friendly preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Add 40 mL of 0.1 mol / L zinc acetate aqueous solution and 200 mL of 0.1 mol / L triethanolamine aqueous solution into an Erlenmeyer flask, stir at room temperature for 30 min, and then irradiate it ultrasonically in a water bath at 30°C for 20 min After that, let it stand for 10h. After centrifugation, washing and drying, ZnO nanospheres are obtained.
[0038] (2) Add 80.0 mg of ZnO nanospheres and 20.0 mg of cobalt nitrate obtained in step 1 to an Erlenmeyer flask containing a mixed solvent of DMF and water (64 mL, volume ratio 3:1), and ultrasonically 20 min at room temperature to fully diffuse , then add 0.660g of 2-methylimidazole, after ultrasonication for 5min, transfer it to a hot water kettle, place it in a 50°C oven for 5h, after the reaction, centrifuge, wash and dry to obtain ZnO@Zn / Co-ZIF precursor.
[0039](3) The ZnO@Zn / Co-ZIF precursor obtained in step 2) was placed in a high-temperature furnace, and the temperature was raised to 800 °C at a rate of 5...
Embodiment 2
[0043] (1) Add 40 mL of 0.1 mol / L zinc acetate aqueous solution and 200 mL of 0.1 mol / L triethanolamine aqueous solution into an Erlenmeyer flask, stir at room temperature for 30 min, and then irradiate it ultrasonically in a water bath at 30°C for 20 min After that, let it stand for 10h. After centrifugation, washing and drying, ZnO nanospheres are obtained.
[0044] (2) Add 80.0 mg of ZnO nanospheres and 12.0 mg of cobalt nitrate obtained in step 1 to an Erlenmeyer flask containing a mixed solvent of DMF and water (64 mL, volume ratio 2:1), and ultrasonicate for 20 min at room temperature to fully diffuse , then add 0.660g of 2-methylimidazole, after ultrasonication for 5min, transfer it to a hot water kettle, put it in a 60°C oven for 4h, after the reaction, centrifuge, wash and dry to get ZnO@Zn / Co-ZIF precursor.
[0045] (3) The ZnO@Zn / Co-ZIF precursor obtained in step 2) was placed in a high-temperature furnace, and the temperature was raised to 900 °C at a rate of 5 ...
Embodiment 3
[0047] (1) Add 40 mL of 0.1 mol / L zinc acetate aqueous solution and 200 mL of 0.1 mol / L triethanolamine aqueous solution into an Erlenmeyer flask, stir at room temperature for 30 min, and then irradiate it ultrasonically in a water bath at 30°C for 20 min After that, let it stand for 10h. After centrifugation, washing and drying, ZnO nanospheres are obtained.
[0048] (2) Add 80.0 mg of ZnO nanospheres and 8.0 mg of cobalt nitrate obtained in step 1 to an Erlenmeyer flask filled with DMF and water mixed solvent (64 mL, volume ratio 1:1), and ultrasonically 20 min at room temperature to make it fully diffuse , followed by adding 1.32g of 2-methylimidazole, after ultrasonication for 5 minutes, pour the solution in the conical flask into a hydrothermal kettle, place it in a 70°C oven for 3 hours, after the reaction, centrifuge, wash and dry to obtain ZnO@Zn / Co-ZIF precursor.
[0049] (3) The ZnO@Zn / Co-ZIF precursor obtained in step 2) was placed in a high-temperature furnace, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com