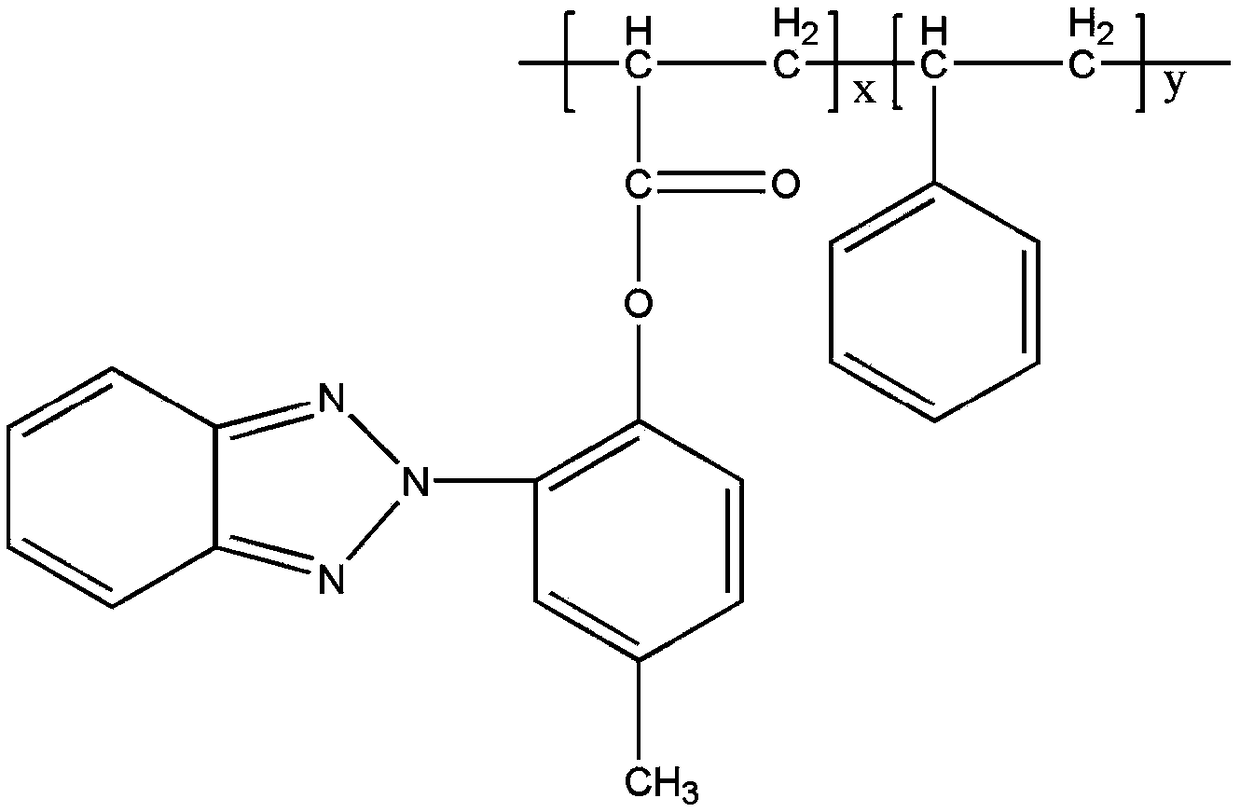
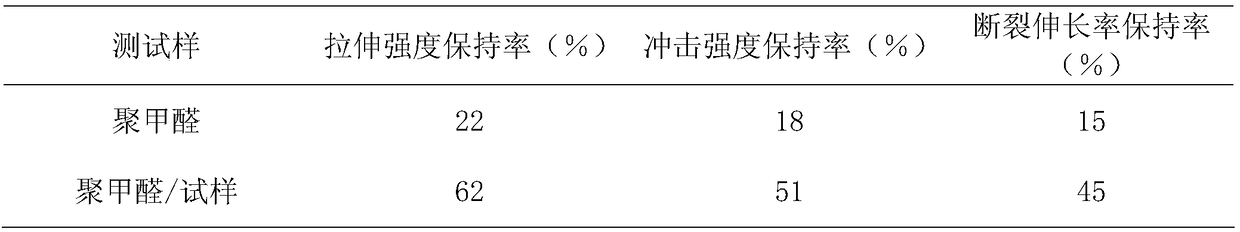
Controllable molecular weight benzotriazole styrene copolymer and its preparation method and application
A technology of benzotriazole styrene and benzotriazole, which is applied in the field of additives for polymer material products, can solve the problems of troublesome control of product molecular weight, difficult control of reaction conditions, and lack of molecular weight, and achieves light stabilization effect. Sustained Effectiveness, Long-lasting Light Stabilization, Simple Operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 2-(2'-propionyloxy-5'-methylphenyl)benzotriazole 3,36g, styrene 2.5g, azobisisobutyronitrile 0.021g, chain transfer agent S, S'- 1 / 3 of the total amount of bis(α,α'-methyl-α″-acetic acid) trithiocarbonate 0.353g is added to the reactor and dissolved in dioxane, fully stirred and dissolved at normal temperature; another 2 / 3 of the chain transfer agent total amount 0.353g in the container is dissolved in dioxane.
[0024] Add 10ml of dioxane, fully stir to dissolve; under the protection of nitrogen, increase the reaction temperature to 60°C, the first stage, start the initial reaction, time 1.0 hours; second stage temperature 70°C, drop the remaining 2 / 3 chain transfer agent solution in the reactor, continue to react, the reaction time is 2.0 hours, in the third stage, after the chain transfer agent is added dropwise, the temperature is raised to 90°C, and the reaction time is 2.5 hours.
[0025] After the reaction is completed, the product mixture is poured into methano...
Embodiment 2
[0028] 2-(2'-propionyloxy-5'-methylphenyl)benzotriazole 3,36g, styrene 2.5g, azobisisobutyronitrile 0.025g, chain transfer agent S, S'- Di(α,α′-methyl-α″-acetic acid) trithiocarbonate 0.212g, 1 / 3 of the total amount, was added to the reactor and dissolved in 10ml of dioxane, fully stirred and dissolved at room temperature; 2 / 3 of the chain transfer agent total amount 0.212g in another container is dissolved in dioxane.
[0029] Add 10ml of dioxane, fully stir to dissolve; under the protection of nitrogen, increase the reaction temperature to 50°C, the first stage, start the initial reaction, time 2.0 hours; second stage temperature 65°C, dropwise add the remaining 2 / 3 chain transfer agent solution in the reactor, continue to react, the reaction time is 4.0 hours, in the third stage, after the chain transfer agent is added dropwise, the temperature is raised to 90°C, and the reaction time is 6.0 hours.
[0030] After the reaction is completed, the product mixture is poured int...
Embodiment 3
[0032] 6.72g of 2-(2'-propionyloxy-5'-methylphenyl)benzotriazole, 1.25g of styrene, 0.025g of azobisisobutyronitrile, chain transfer agent S, S'-di (α, α'-methyl-α"-acetic acid) 1 / 3 of the total amount of 0.216g trithiocarbonate is added to the reactor and dissolved in 15ml of dioxane, fully stirred and dissolved at normal temperature; another 2 / 3 of the chain transfer agent total amount 0.216g in the container is dissolved in dioxane.
[0033] Under the protection of nitrogen, raise the reaction temperature to 50°C. In the first stage, the initial reaction starts for 0.5 hours; in the second stage, the temperature is 70°C, and the remaining 2 / 3 chain transfer agent solution is added dropwise to the reactor , continue the reaction, the reaction time is 3.0 hours, in the third stage, after the dropwise addition of the chain transfer agent is completed, the temperature is raised to 85° C., and the reaction time is 2.5 hours.
[0034]After the reaction is completed, the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com