Application of manganese oxide-fullerene hybrid material in near-infrared light denitrification
A near-infrared light and fullerene technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1)C 60 -α-MnO 2 Preparation: Weigh manganese chloride and potassium permanganate (molar ratio of 3:2), and dissolve them in 20 mL of deionized water respectively, and add 5% fullerenes of the theoretical yield of manganese dioxide to the manganese chloride solution. , transferred to a three-necked flask by ultrasonic for 1 hour, heated to 85°C in a water bath, and added dropwise with potassium permanganate, refluxed into a condenser for 12h, filtered and washed, and vacuum-dried at 70°C for 12 hours to obtain the fullerene. -α-Manganese dioxide nanocomposite photocatalyst.
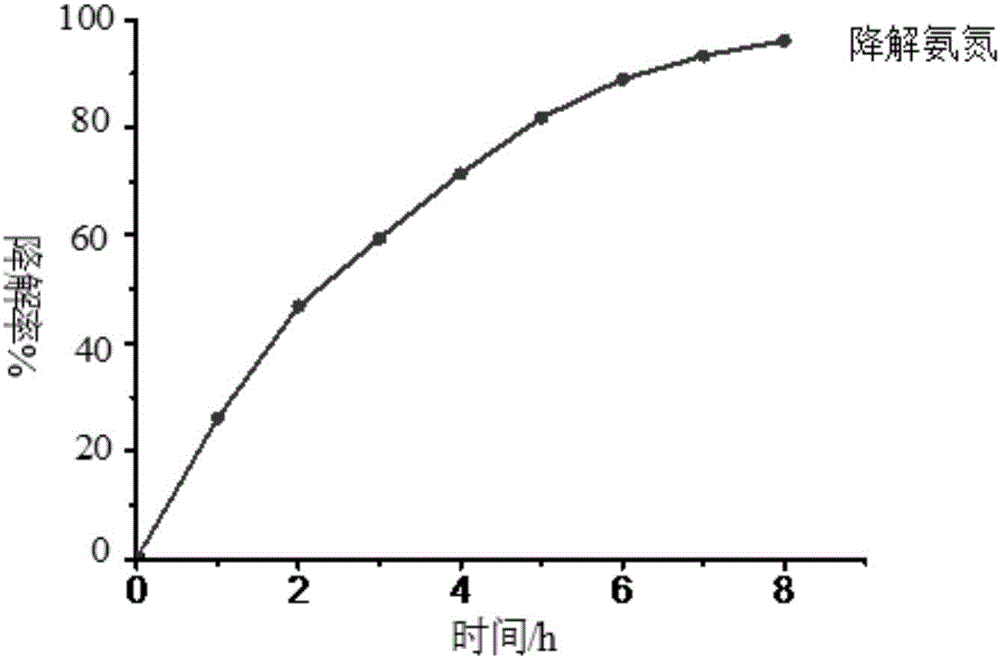
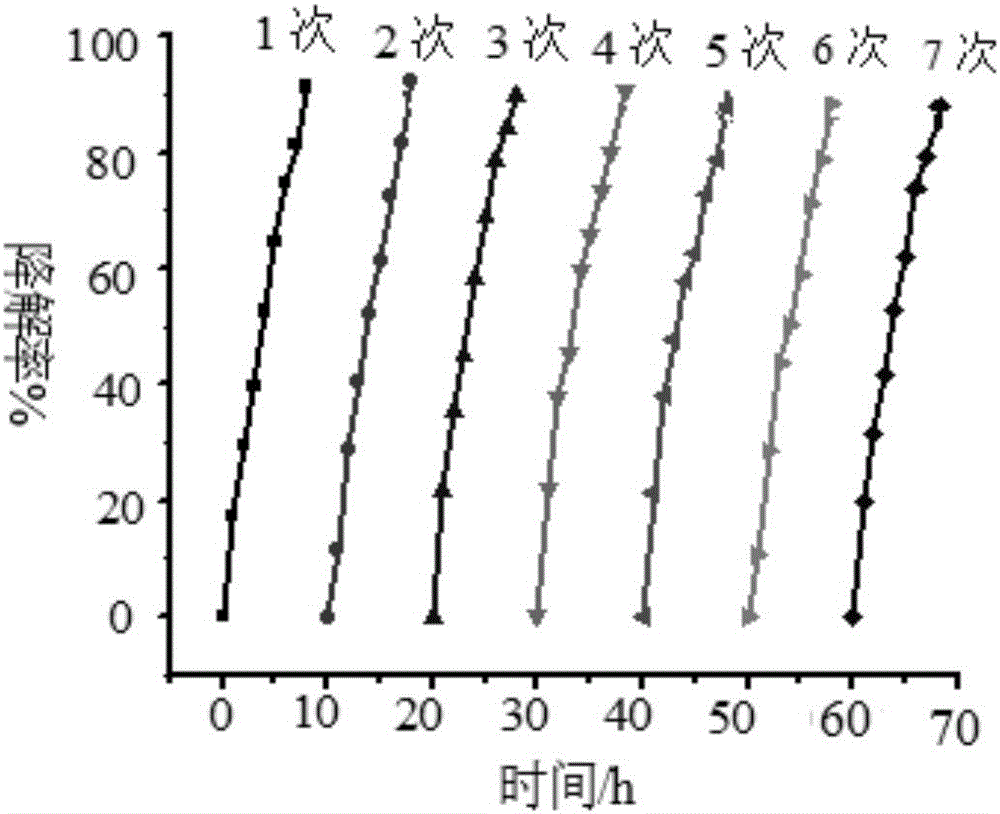
[0039] (2) Photocatalysis experiment: wrap the wall of a 100ml beaker with tin foil to prevent ultraviolet light and visible light from entering the reaction system, and cover the beaker mouth with a λ>780nm cut-off filter to ensure that only near-infrared light The radiation entered the photoreactor, and a 300W UV-Vis lamp was placed above the reactor. Add a certain concentration of ammonia nit...
Embodiment 2
[0045] C 60 -β-MnO 2 Preparation: Weigh manganese chloride and potassium permanganate (molar ratio of 3:2), dissolve them in 20 mL of deionized water respectively, then add 5% fullerenes of the theoretical yield of manganese dioxide to the manganese chloride solution, ultrasonically For 1 hour, add potassium permanganate solution, stir for 30min, transfer to a 100ml reaction kettle, heat at 160°C for 12h, cool to room temperature, filter and wash, and dry under vacuum at 70°C for 12h to obtain the fuller En-β-manganese dioxide nanohybrid photocatalysts.
[0046] (2) Photocatalysis experiment: wrap the wall of a 100ml beaker with tin foil to prevent ultraviolet light and visible light from entering the reaction system, and cover the beaker mouth with a λ>780nm cut-off filter to ensure that only near-infrared light The radiation entered the photoreactor and a 300W UV-Vis lamp was placed above the reactor. Add a certain concentration of ammonia nitrogen solution to the beaker,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com