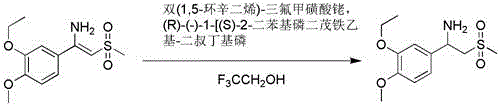
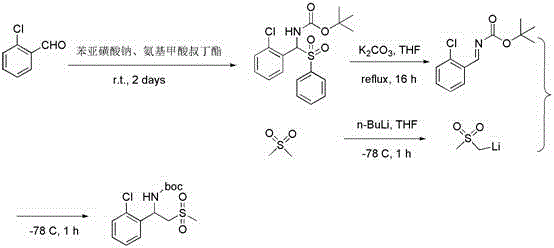
Preparation method of 2-(methyl sulphonyl)-1-aromatic ethylamine
A technology of arylethylamine and methylsulfonyl, applied in the field of pharmaceutical chemical synthesis, can solve the problems of inconvenient production, high risk and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the synthetic technique of 2-(methylsulfonyl)-1-phenylethylamine
[0014] Add 60 ml of methanol, 13.0 g of 2-(methylsulfonyl) acetic acid and 18.2 g of ammonium acetate into a 500 ml three-necked flask, heat to 45°C, add 10.2 g of benzaldehyde dropwise, and heat to reflux after dropping. Cool to below 10°C, add 34.0 g of thionyl chloride dropwise, after the drop is complete, heat to reflux for 17 h, and distill off the methanol. Add 50 ml of dichloromethane to the residue, and adjust the pH to 6~7 with 5 mol / L NaOH solution. The organic layer was separated, and the aqueous layer was extracted with dichloromethane. The organic layers were combined and concentrated to obtain 10.3 g of a colorless oil, with a HPLC purity of 98% and a yield of 61%. 1H NMR (DMSO-d6) δ 1.32 (t, J=7.0 Hz, 3H), 2.08 (s, 2H), 2.96 (s,3H), 3.23 (dd, J=3.6, 14.4 Hz, 1H), 3.41 ( dd, J=9.4, 14.4 Hz, 1H), 3.73 (s,3H), 4.02 (q, J=7.0 Hz, 2H), 4.26 (dd, J=3.7, 9.3 Hz, 1H), 6.89 (s, 2H ...
Embodiment 2
[0015] Embodiment 2: the synthesis technique of 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethylamine
[0016] Add 50 ml of ethanol, 12.4 g of 2-(methylsulfonyl) acetic acid and 18.2 g of ammonium acetate to a 500 ml three-necked flask, heat to 50 °C, add 10.0 g of 3-ethoxy-4-methoxybenzaldehyde dropwise, After dropping, heat to reflux for reaction. Cool to below 10°C, add 40 g of thionyl chloride dropwise, heat to reflux for 16 hours, and distill off ethanol. Add 50 ml of dichloromethane to the residue, and adjust the pH to 6~7 with 5 mol / L NaOH solution. The organic layer was separated, and the aqueous layer was extracted with dichloromethane. The organic layers were combined and concentrated to give 14.2 g of a colorless oil, with a HPLC purity of 98%, and a yield of 78%. 1 H NMR (DMSO- d 6 ) δ 1.32 (t, J =7.0 Hz, 3H), 2.08 (s,2H), 2.96 (s, 3H), 3.23 (dd, J =3.6, 14.4 Hz, 1H), 3.41 (dd, J =9.4, 14.4 Hz,1H), 3.73 (s, 3H), 4.02 (q, J =7.0 Hz, 2H), 4.26 (dd, J =3...
Embodiment 3
[0017] Embodiment 3: the synthesis technique of 1-(3-trifluoromethylphenyl)-2-(methylsulfonyl)ethylamine
[0018] Add 75 ml of methanol, 13.0 g of 2-(methylsulfonyl) acetic acid and 10.9 g of ammonium acetate to a 500 ml three-necked flask, heat to 50 °C, add 10.0 g of 3-trifluoromethylbenzaldehyde dropwise, and heat to reflux reaction. Cool to 0~5°C, add 55 g of thionyl chloride dropwise, heat to reflux for 17 h, and distill off methanol. 50ml of dichloromethane was added to the residue, and the pH was adjusted to 6~7 with 5N NaOH solution. The organic layer was separated, and the aqueous layer was extracted with dichloromethane. The organic layers were combined and concentrated to give 9.8 g of a colorless oil, HPLC purity 98%, yield 58%, 1H-NMR (400 MHz, DMSO-d6): δ = 3.01 (s, 3H), 3.84 (dd, 1H) , 4.03 (dd,1H), 5.01 (t, 1H), 7.71 (t, 1H), 7.82 (d, 1H), 7.91 (d, 1H), 8.03 (s, 1H),8.75 (br. s., 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com