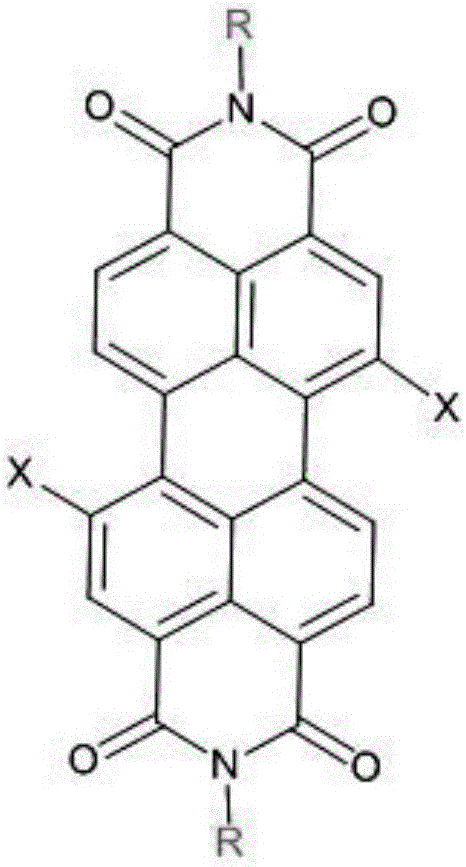
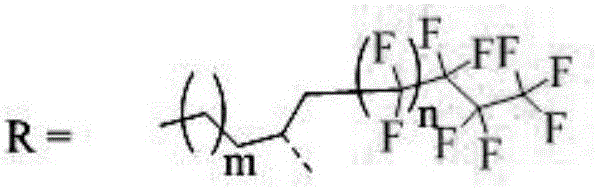
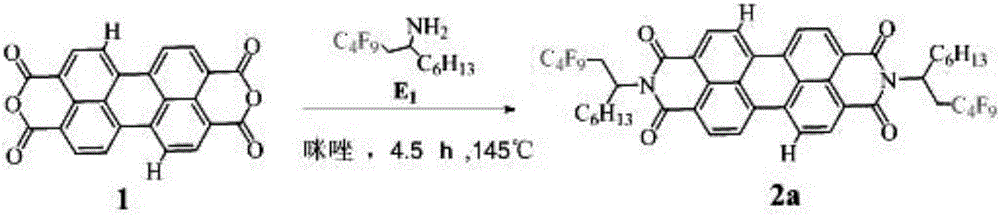
Perfluoroalkyl-modified perylene imide and its preparation method
A technology of perfluoroalkyl and perylene imide, applied in the field of perfluoroalkyl modified perylene imide and its preparation, can solve the problems of poor solubility of perylene imide, unsuitable for solution processing, etc. Thermal stability, the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of N,N'-bis(2-perfluorobutyl-1-hexylethyl)peryleneimide
[0017]
[0018] 25mL two-neck flask with perylene tetracarboxylic anhydride 1 (0.72mmol, 0.281g), E 1 (2.45mmol, 0.852g), imidazole (58.76mmol, 4g), react at 145°C for 4.5h under anaerobic conditions, cool to 120°C, add appropriate amount of ethanol, reflux for 0.5h, add 12mol / L HCl to remove imidazole, use CH 2 Cl 2 Extract with saturated brine, dry with anhydrous magnesium sulfate, filter with suction, rotary steam, use CH 2 Cl 2 Using petroleum ether as the eluent, silica gel was passed through a column for separation, to obtain 0.152 g of perfluoroalkyl perylene imide 2 with a yield of 20.19%. 1 H NMR(400MHz, CDCl 3 )δ8.74(s, 2H), 8.65(s, 6H), 5.73(s, 2H), 3.36(d, 2H), 2.55(s, 2H), 2.39(s, 2H), 1.93(s, 2H) ),1.24(s,8H),0.83(s,6H); HRMS(MALDI-TOF):Calcd for C 48 H 32 F 26 N 2 O 4 ,1194.1950,found:1194.7520(M - ).
Embodiment 2
[0019] Example 2: Preparation of N,N'-bis(2-perfluorobutyl-1-hexylethyl)peryleneimide
[0020]
[0021] Add perylene tetracarboxylic anhydride 1 (0.678mmol, 0.266g), E1 (1.695mmol, 0.589g), CH3COOH (10mL) in a 25mL two-neck flask, react at 115°C for 6.5h under anaerobic conditions, and extract with CH2Cl2 and saturated brine , Dried over anhydrous magnesium sulfate, filtered with suction, rotary steamed, used CH2Cl2 and petroleum ether as eluents, separated by silica gel column to obtain perfluoroalkyl perylene imide 2a 0.256 g, yield: 35.93%. 1H NMR(400MHz,CDCl3)δ8.73(d,2H),8.64(s,6H),5.72(s,2H),3.35(s,2H),2.56(s,2H),2.35(s,2H) , 1.91 (s, 2H), 1.28 (d, 16H), 0.82 (s, 6H); HRMS (MALDI-TOF): Calcd for C48H40F18N2O4, 1050.2700, found: 1050.8290 (M-).
Embodiment 3
[0022] Example 3: Preparation of N,N'-bis(2-perfluorohexyl-1-hexylethyl)peryleneimide
[0023]
[0024] 25mL two-neck flask with perylenetetracarboxylic anhydride 1 (0.77mmol, 0.301g), E 2 (1.49mmol, 0.67g), imidazole (73.45mmol, 5g), react at 145°C under anaerobic conditions for 6.5h, cool to 120°C, add appropriate amount of ethanol, reflux for 0.5h, add 12mol / L HCl to remove imidazole, use CH 2 Cl 2 Extract with saturated brine, dry with anhydrous magnesium sulfate, filter with suction, rotary steam, use CH 2 Cl 2 Using petroleum ether as eluent, silica gel column separation, 0.278 g of perfluoroalkyl perylene imide 2b, yield: 29%. 1 H NMR(400MHz, CDCl 3 )δ8.73(s, 2H), 8.65(s, 6H), 5.73(s, 2H), 3.36(d, 2H), 2.55(s, 2H), 2.36(s, 2H), 1.94(s, 2H) ),1.24(s,16H),0.83(s,6H); HRMS(MALDI-TOF):Calcd for C 52 H 40 F 26 N 2 O 4 ,1250.2570,found:1250.8600(M - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com