Phthalocyanine-aryl ruthenium compound and preparation method and application thereof
A ruthenium compound and aryl technology, applied in the field of monochloro-β-1-2-ethoxy-phthalocyanine-methylisopropylphenylruthenium and its preparation, can solve the problem of no tumor inhibitory effect and cross drug resistance In order to achieve the effects of low cost, easy-to-obtain raw materials and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
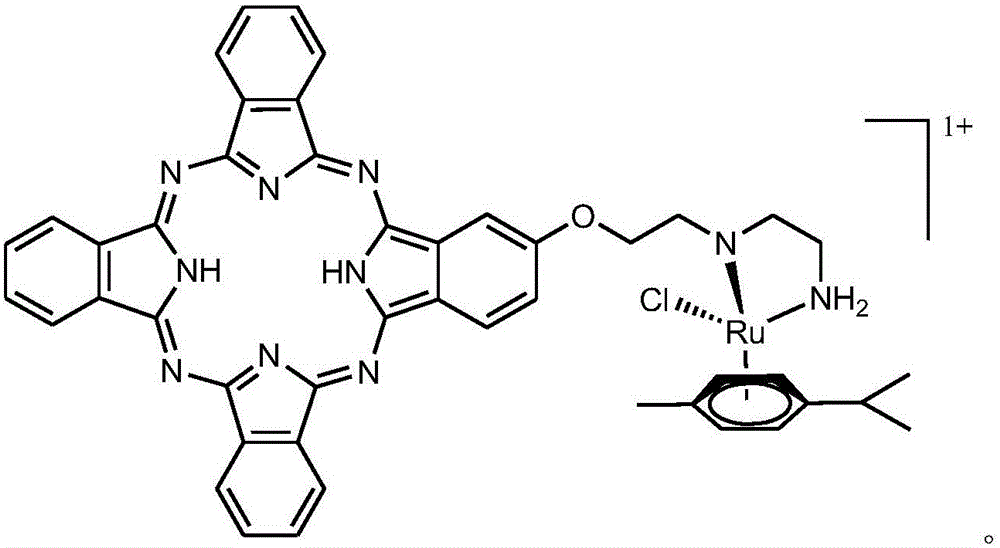
[0031] A phthalocyanine-arylruthenium compound:
[0032] 1) The chemical name of the phthalocyanine-arylruthenium compound is monochloro-β-one-2-(2-diethylamine)ethoxy-phthalocyanine-methylisopropylphenylruthenium(II);
[0033] Structural formula:
[0034]
[0035] 2) Physical and chemical properties: monochloro-β-1-2-(2-diethylamine)ethoxy-phthalocyanine-methylisopropylphenylruthenium(II), red crystal, easily soluble in water and organic solvent, its H NMR spectrum data is 1 H NMR: (ppm, CDCl 3 )δ=0.97(3H,s),1.13(6H,d,J=6.9Hz),2.35(3H,s),2.77-2.97(6H,m,J=6.1Hz),3.12(1H,m,J = 6.9Hz), 3.46 (2H, d, J = 5.9Hz), 6.77-7.90 (15H, m, J = 6.1Hz).
Embodiment 2
[0037] The preparation method of a chloro-β-one-2-(2-diethylamine) ethoxy-phthalocyanine-methyl isopropyl phenyl ruthenium (II), comprises the following steps:
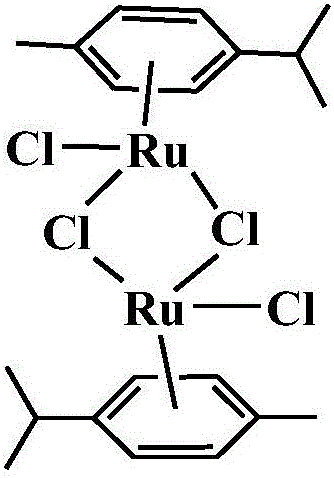
[0038] 1) 0.366g ruthenium weight content is 37% RuCl 3 ·xH 2 O and 3ml of γ-terpinene with a purity of 95% were dissolved in 10ml of absolute ethanol, heated to reflux and stirred for 6 hours, and left to separate out to obtain dichloro-dichloro-bis-methylcumene diruthenium (II) .
[0039]
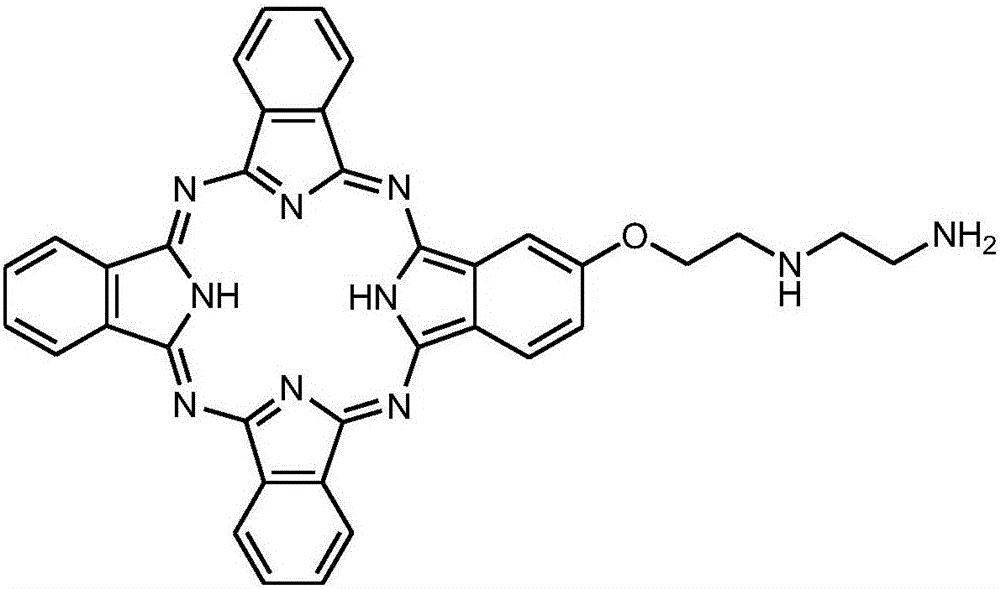
[0040] 2) Weigh 0.095g of β-1-nitro-phthalocyanine and 0.12g of 2-(2-diethylamine)ethanol, dissolve them in 10ml of DMF solution, react at 80°C for 6 hours under the protection of nitrogen, and then stand to precipitate to obtain β- Mono-2-(2-diethylamine)ethoxy-phthalocyanine.
[0041]
[0042] 3) Dissolve 30 mg of β-one-2-(2-diethylamine) ethoxy-phthalocyanine and 12 mg of dichloro-di-methylcumene diruthenium (II) in 8 ml of Water and ethanol, heated and stirred to reflux for 6 hours. After the reaction was completed,...
Embodiment 3
[0044] In vitro cytotoxicity assays were performed using the MTT method. The organometallic ruthenium compound obtained in Example 1 was reacted with the ovarian cancer A2780 cell line for 72 hours respectively, and the results are shown in Table 1.
[0045] Table 1 organometallic ruthenium compounds to the half maximal effective concentration (IC) of tumor cell lines 50 )
[0046] cell line A2780 (protect from light) A2780 (illumination) IC 50 (μmol / mL)
6.0±0.8 1.3±0.3
[0047] In vitro tumor cell inhibitory activity experiments show that a chlorine-beta-one-2-(2-diethylamine) ethoxy-phthalocyanine-methylisopropylphenylruthenium (II) compound of the present invention is effective on ovarian cancer The A2780 cell line has a significant inhibitory effect, and its inhibitory effect is significantly enhanced under visible light irradiation.
[0048] From the above results, it can be seen that the phthalocyanine-aryl ruthenium compound of the present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com