Device for treating lung of patient
A patient and mesh stent technology, applied in the field of medical devices, can solve the problems of Mycobacterium tuberculosis residues, puncture complications, multidrug-resistant cavitary tuberculosis patients who cannot be cured, and achieve the effect of promoting cavity closure and negative conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] In this embodiment, the device for treating a patient's lung mainly includes an implant, and the implant is composed of a support protection member and a membrane member.
[0066] In this embodiment, the proximal end refers to the end close to the operator, and the distal end refers to the end away from the operator.
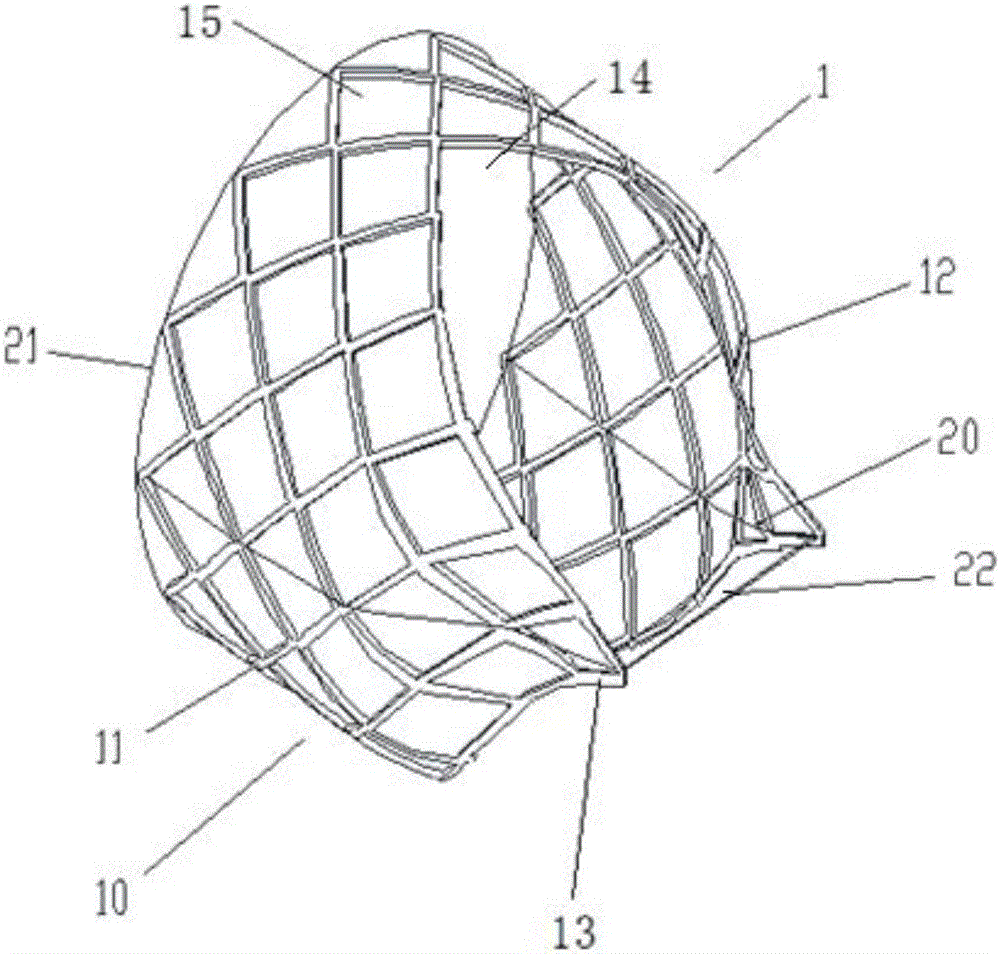
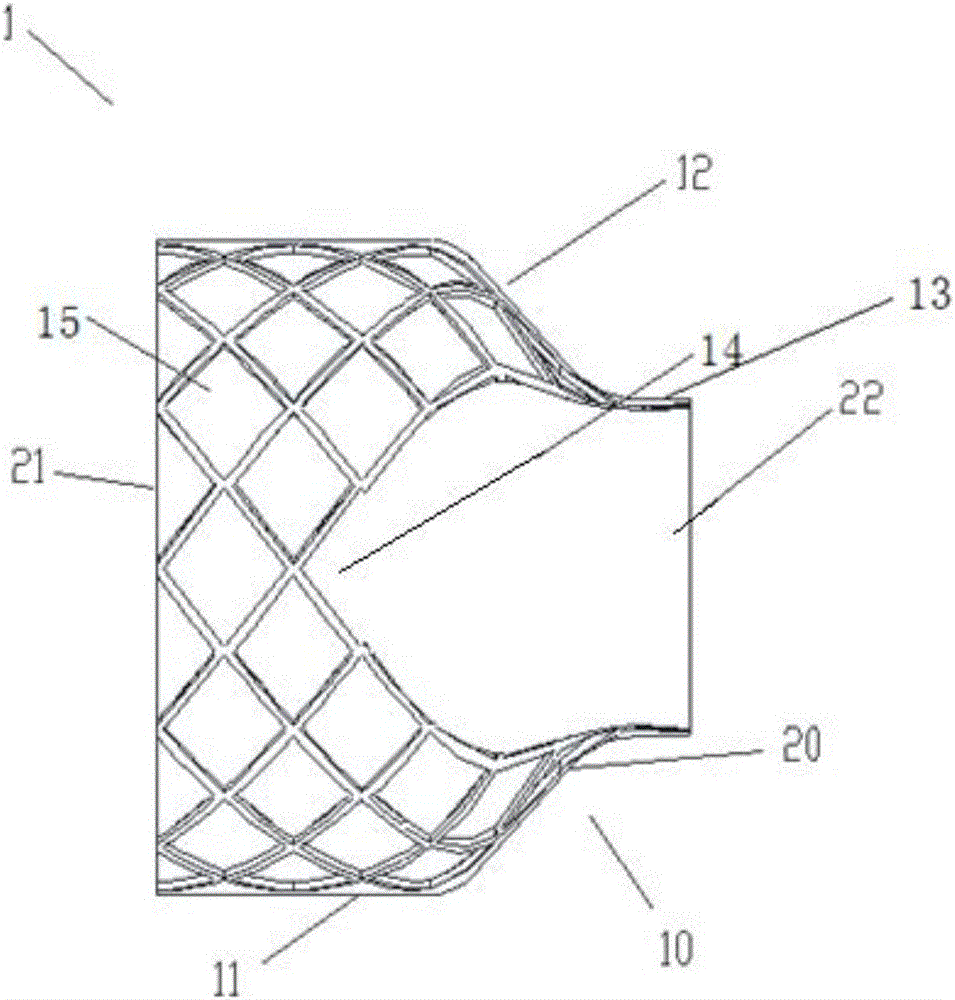
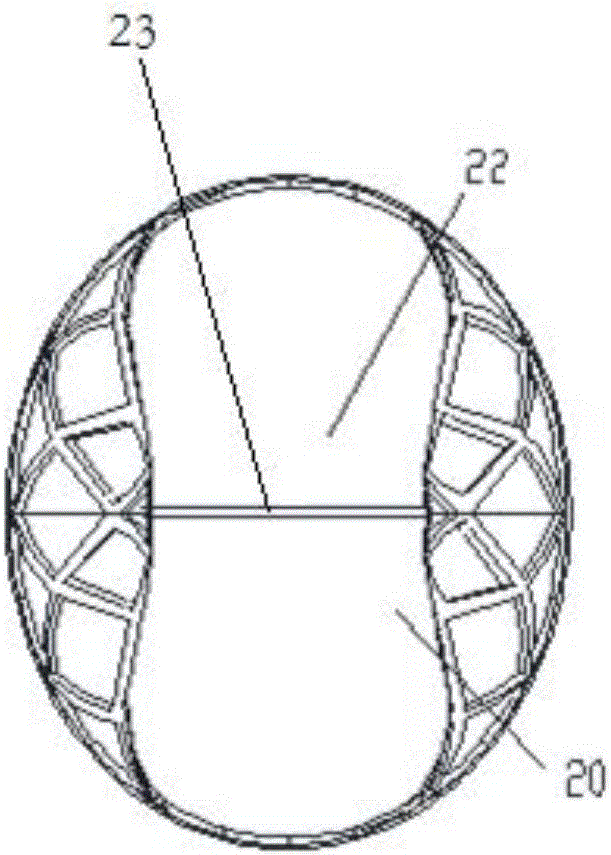
[0067] Figure 1-Figure 4 is a schematic structural view of the implant 1 according to this embodiment. See Figure 1-Figure 3 As shown, the implant 1 of this embodiment is composed of a mesh stent 10 and a valve 20 attached to the inner surface of the mesh stent 10; wherein, the mesh stent 10 includes a supporting part 11, a protecting part 13 and a The smooth transition portion 12; the valve 20 includes a valve edge 21 and a leaflet 22; see image 3 There is a wedge-shaped opening 23 between the two leaflets 22, and the ends of the two leaflets 22 are in a closed initial shape. The wedge-shaped openings 23 at the ends of the two leaflets 22 realize t...
Embodiment 2
[0089] In this embodiment, the device for treating a patient's lung mainly includes an implant, and the implant is composed of a support protection member and a membrane member. In this embodiment, the proximal end refers to the end close to the operator, and the distal end refers to the end away from the operator.
[0090] Figure 8 is a schematic structural view of the implant according to this embodiment. Such as Figure 8 As shown, the implant 1 of this embodiment includes a mesh stent 10 and a valve 20, the mesh stent 10 includes a support portion 11, a protection portion 13, and a smooth transition portion 12 between the support portion 11 and the protection portion 13 . The mesh stent 10 is a mesh-shaped hollow stent composed of a plurality of closed polygonal grids 15 , and the mesh-shaped hollow design of the mesh stent 10 is more conducive to the attachment of the valve 20 to the mesh stent 10 .
[0091] The mesh stent 10 can be made of a tube body made of elasti...
Embodiment 3
[0102] In this embodiment, the device for treating a patient's lung mainly includes an implant, and the implant is composed of a support protection member and a membrane member. And in this embodiment, the proximal end refers to the end close to the operator, and the distal end refers to the end far away from the operator.
[0103] Figure 14 is a schematic structural view of the implant according to this embodiment. Such as Figure 14 As shown, the implant 1 of this embodiment includes a mesh stent 10 whose diameter becomes smaller after being compressed in a direction perpendicular to the axial direction and returns to its original shape after decompression. The mesh stent 10 includes a support portion 11 and a protection portion 13 and the smooth transition portion 12, the implant body 1 is provided with a plurality of U-shaped openings 14 at the edge position of the support portion 11, and a valve 20 is attached to the inner surface of the implant body 1 corresponding to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com