Application of diaryl ketone compounds in preparation of antitumor drug
A technology of diaryl ketones and anti-tumor drugs, which is applied in the field of application of diaryl ketone compounds in the preparation of anti-tumor drugs, and can solve problems such as evaluation of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
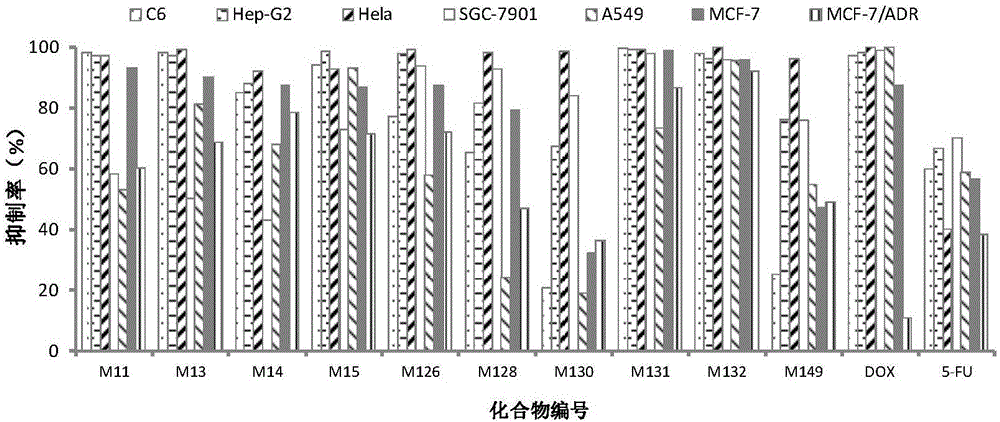
[0022] In the following, unless otherwise specified, the materials and methods of operation used in the present invention are well known in the art. [Example 1] Evaluation of in vitro antitumor activity of compounds
[0023] Tested tumor cells: human hepatoma cell HepG2, human lung cancer cell A549, human breast cancer cell MCF-7, doxorubicin-resistant human breast cancer cell (MCF-7 / ADR), human gastric cancer cell SGC-7901, human cervical cancer Cell Hela, human glioma cell C6.
[0024] Cell culture: GIBCO DMEM medium, 10% fetal bovine serum and 0.01% L-glutamine were formulated into culture medium. Cultured cell lines were placed at 37°C, 5% CO. 2 The cells were routinely cultured and passaged under saturated humidity, and the cells in the logarithmic growth phase were used in the experiments.
[0025] In vitro antitumor activity evaluation (MTT method):
[0026] The above tumor cells were plated in 96-well plates, and after they were cultured in a 37°C, 5% CO2 incubator...
Embodiment 2
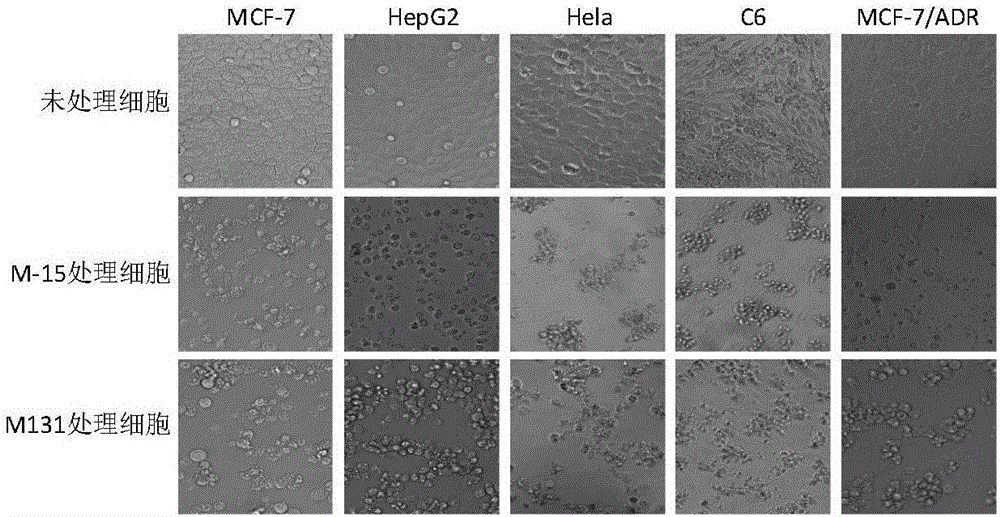
[0036] [Example 2] Tumor cytopathic effects caused by compounds
[0037] We further recorded the cytopathic effect of Hela, C6, HepG2, MCF-7, MCF-7 / ADR tumor cells induced by representative compounds M131 and M15 by microscopy. The specific implementation method is as follows:
[0038] Hela, C6, HepG2, MCF-7, MCF-7 / ADR tumor cells in logarithmic growth phase were plated in 24-well plates, and the cell culture medium was discarded after culturing at 37°C in a 5% CO2 incubator. , respectively, adding 20 μg / mL M131 and 40 μg / mL M15 test compound cell maintenance solution to continue culturing, and 48 hours later, the cytopathic conditions were observed and photographed by microscope.
[0039] Compound-induced tumor cytopathic effects such as image 3 shown. Under the microscope, untreated tumor cells grew well, adhered firmly, with plump shapes and clear borders. Treatment of 20μg / mL M131 and 40μg / mL M15 for 48h resulted in apoptosis of all tumor cells, and the cells became r...
Embodiment 3
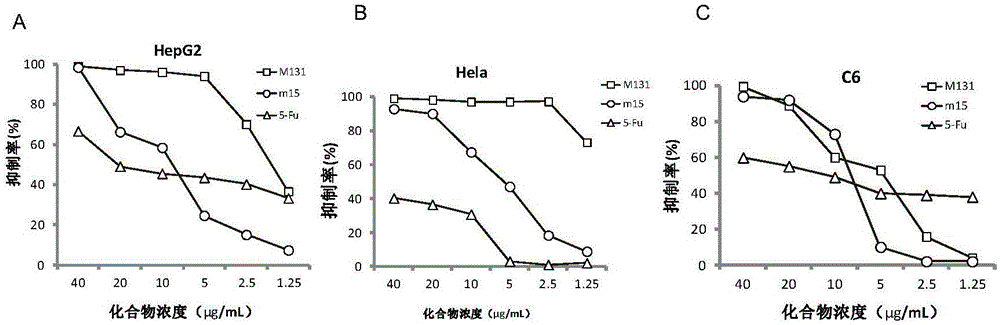
[0040] [Example 3] Evaluation of tumor cell apoptosis activity induced by compounds
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com