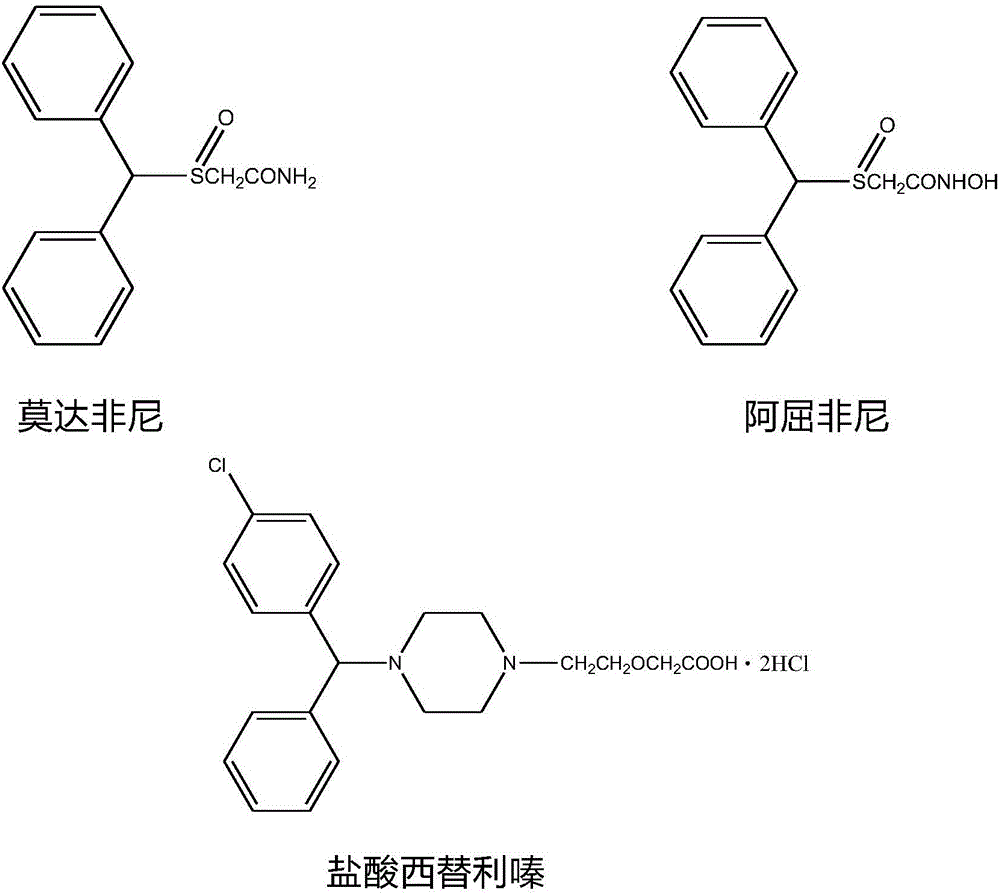
Synthetic method of diaryl methanol compound
The technology of a diaryl methanol and a synthesis method is applied in the environmental friendly technical field, can solve the problems of low yield, high toxicity of aryl formaldehyde, etc., and achieves the effects of easy separation and purification, safe and stable raw materials, and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of diphenylcarbinol
[0042]
[0043] Add 1mmol of benzyl bromide, 1.2mmol of phenylboronic acid, 2mmol of anhydrous potassium carbonate, 2.5mmol of PEG2000, 0.01mmol of catalyst, 5ml of pure water into the reaction flask, and react for 4 hours in the air at room temperature, stop the reaction, and divide with about 40ml of ethyl acetate. Extract three times to obtain the organic phase of ethyl acetate, add 2-3 tablespoons of anhydrous sodium sulfate and let stand for 4 hours to remove water, filter and rotary evaporate, use TLC method to separate, use (n-hexane:dichloromethane=7:1, v : v) The product was isolated as a developer, and recrystallized with ethanol to obtain diphenylmethanol with a yield of 80%.
Embodiment 2
[0044] Embodiment 2: the preparation of two (4-fluorophenyl) methanol
[0045]
[0046] Add 1mmol of 4-fluorobenzyl bromide, 1.2mmol of 4-fluorophenylboronic acid, 2mmol of anhydrous potassium carbonate, 2.5mmol of PEG2000, 0.01mmol of catalyst, 5ml of pure water into the reaction flask, and react for 4h at room temperature in air, stop the reaction, and use About 40ml of ethyl acetate was extracted three times to obtain the organic phase of ethyl acetate, add 2-3 spoons of anhydrous sodium sulfate and let it stand for 4h to remove water, filter and rotary evaporate, use TLC method to separate, use (n-hexane: dichloromethane =7:1, v:v) The product was isolated as a developer and recrystallized with ethanol to obtain bis(4-fluorophenyl)methanol with a yield of 75%.
Embodiment 3
[0047] Embodiment 3: the preparation of p-methylbenzhydryl alcohol
[0048]
[0049] Add 1mmol of 4-methylbenzyl bromide, 1.2mmol of phenylboronic acid, 2mmol of anhydrous potassium carbonate, 2.5mmol of PEG2000, 0.01mmol of catalyst, and 5ml of pure water into the reaction flask, and react for 4 hours in the air at room temperature, stop the reaction, and use about 40ml Ethyl acetate was extracted three times to obtain the organic phase of ethyl acetate. Add 2-3 spoons of anhydrous sodium sulfate and let it stand for 4 hours to remove water. Suction filtration and rotary steaming were used for separation by TLC. : 1, v: v) The product was isolated as a developer, and recrystallized with ethanol to obtain p-methylbenzhydryl alcohol with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com