Synthetic method for sotagliflozin and its analogues
A synthetic method and analog technology, applied in the field of drug synthesis, can solve the problems of lengthy synthetic routes, reduced synthetic efficiency, high price, etc., and achieve the effects of simplifying experimental operations, stabilizing water and air, and reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
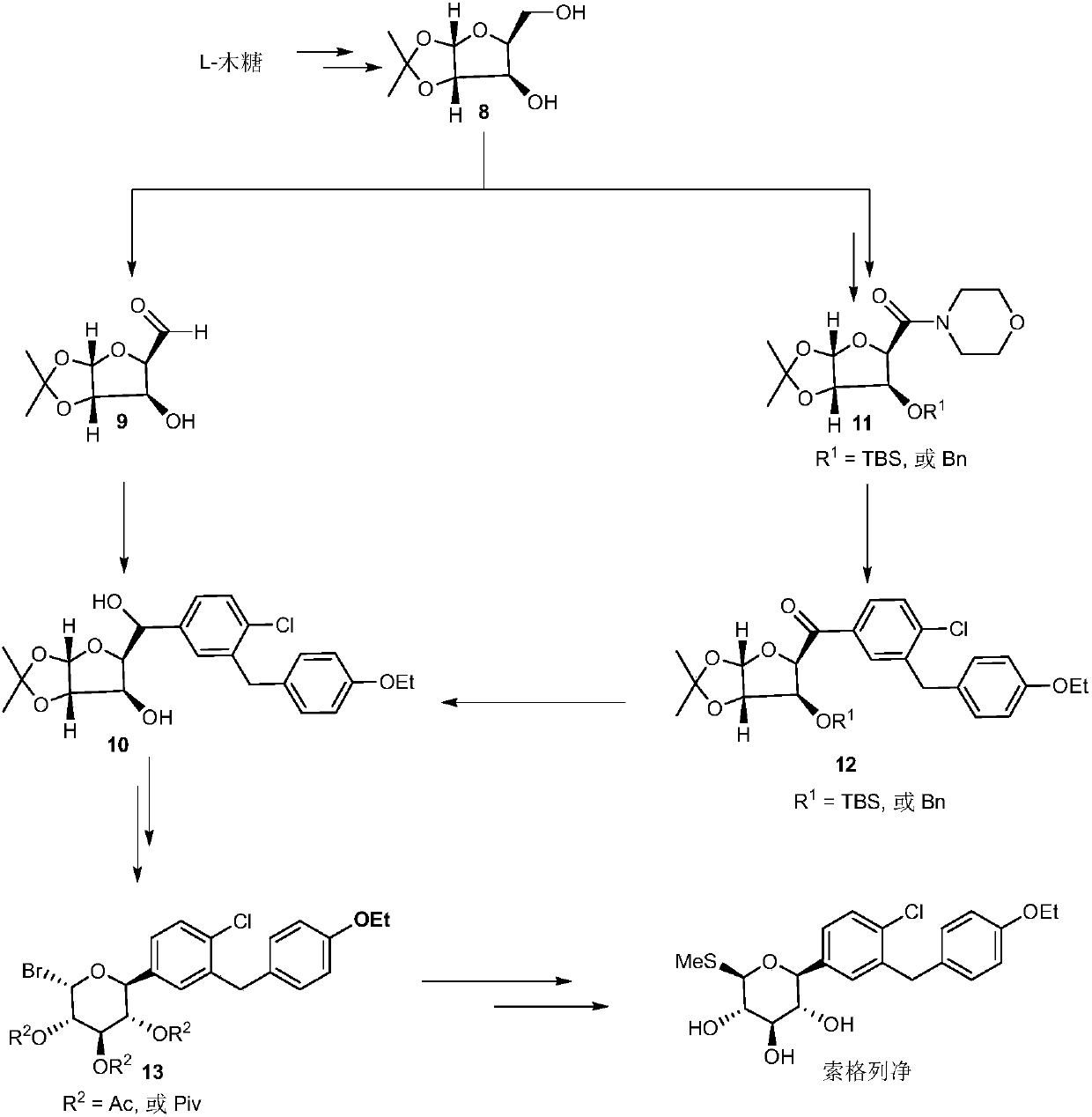
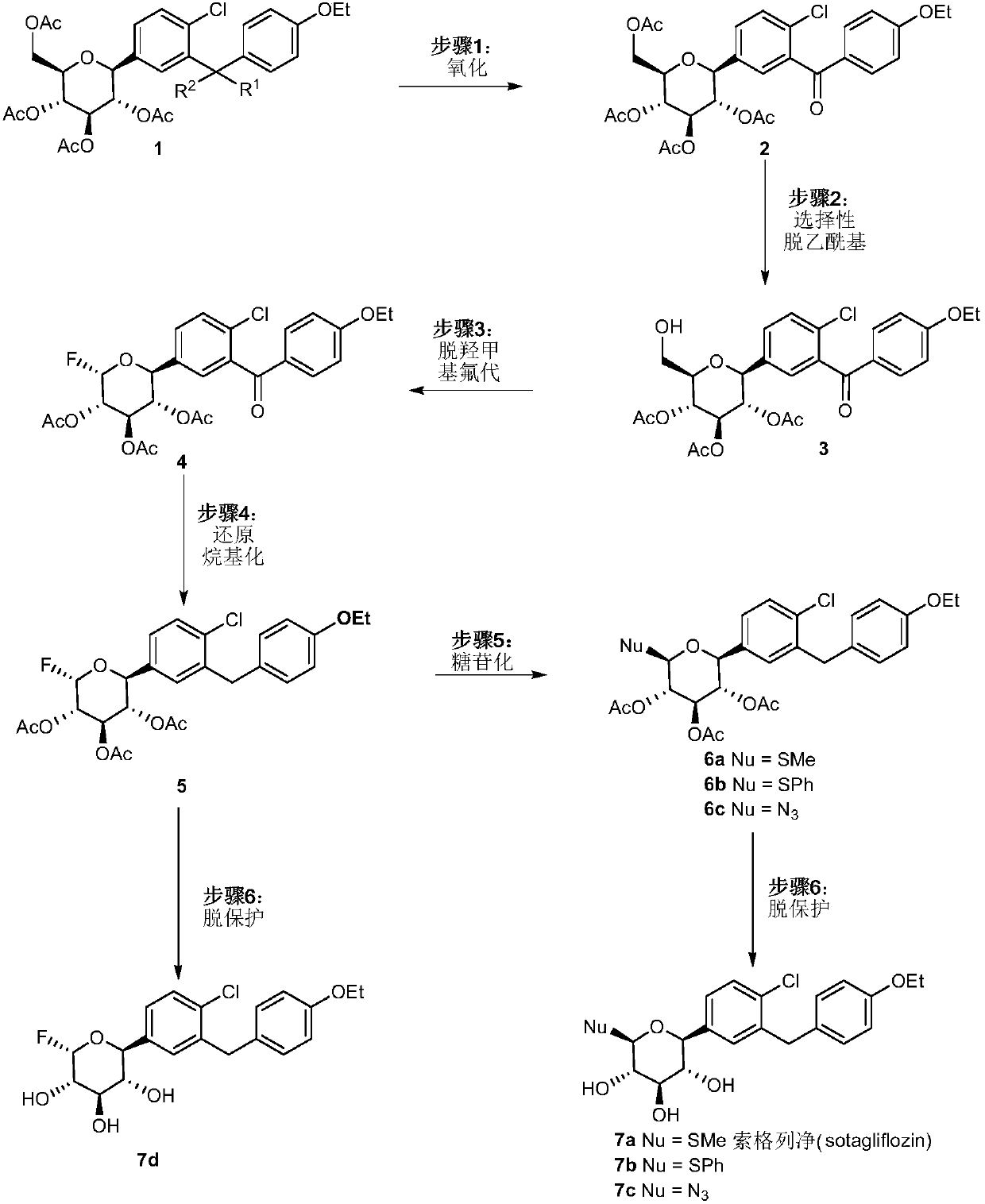
[0047] The following takes the synthesis of soxagliflozin and its partial analogues as an example to describe the present invention in detail, but the present invention is not limited to the following content.
[0048] (1) Preparation and data of compound 2:
[0049] Under argon protection, add dapagliflozin acetate 1 (500mg, 0.87mmol, 1eq; J.Med.Chem.2008,51,1145-1149) into a 100mL dry reaction bottle equipped with a magnetic stirrer, carbonic acid Silver (122mg, 0.44mmol, 0.5eq) and 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2,2,2]-octane-bis(tetrafluoroborate) salt (1.5g , 4.35mmol, 5eq), dissolved in 35mL of acetone / water (v / v=6 / 1), ventilated 3 times, and reacted at 15°C. After 24h, the reaction was complete, washed with water, washed with saturated saline solution, CH 2 Cl 2 Extract, combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, separate and purify by column chromatography (petroleum ether: ethyl acetate = 3:1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com