Method for preparing bis (indolyl) methane derivative in presence of amine salt
A technology of bisindolylmethane and indole derivatives, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, long reaction time and high substrate requirements, and achieves simple operation, high reaction efficiency and good substrate applicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
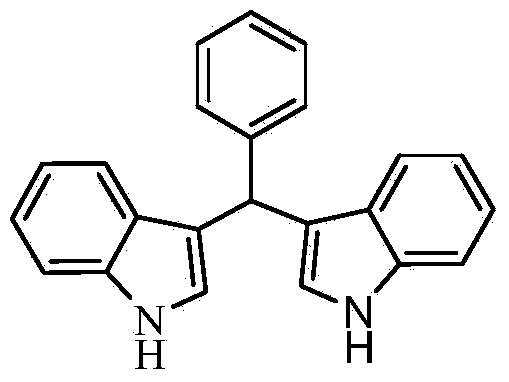
[0011] Taking the preparation of the following formula compound bis-indolephenylmethane as an example, the raw materials used and the preparation method thereof are as follows:
[0012]
[0013] Add 0.106g (1.0mmol) of benzaldehyde, 0.234g (2.0mmol) of indole, and 0.0165g (0.1mmol) of o-chloroaniline hydrochloride into 1mL of acetonitrile, and react at room temperature for 10 minutes to stop the reaction. The mixed solution with ether volume ratio of 3:1 was separated by mobile phase column chromatography to obtain bright pink solid bisindolephenylmethane with a yield of 99%. The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (400MHz, DMSO) δ: 10.83 (d, J = 1.3Hz, 2H), 7.46-7.20 (m, 8H), 7.15 (d, J = 7.3Hz, 1H), 7.04 (dd, J = 11.1, 4.0Hz, 2H), 6.92-6.78(m, 4H), 5.86(s, 1H); 13 C NMR (101 MHz, DMSO) δ: 144.98, 136.63, 128.32, 128.01, 126.67, 125.76, 123.56, 120.89, 119.14,...
Embodiment 2
[0015] In Example 1, o-chloroaniline hydrochloride was replaced with equimolar aniline hydrochloride, and reacted at room temperature for 30 minutes. The other steps were the same as in Example 1 to prepare pink solid bis-indolephenylmethane. The yield 98%.
Embodiment 3
[0017] In Example 1, o-chloroaniline hydrochloride was replaced with equimolar aniline trifluoroacetate, and reacted at room temperature for 30 minutes. The other steps were the same as in Example 1, and a pink solid bis-indolephenylmethane was prepared. The yield was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com