Synthesis method and application of axially chiral 9-aryl tetrahydroacridine
A technology for the synthesis of aryl tetrahydroacridines, which is applied in the fields of organic chemistry and organic chemistry, can solve the problems of small C-C bond rotation energy barrier, loss of drug development field, and inability to form anti-rotational chirality, etc., to achieve technological process The effect of short, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Preparation of axial chiral 9-aryl tetrahydroacridine compounds
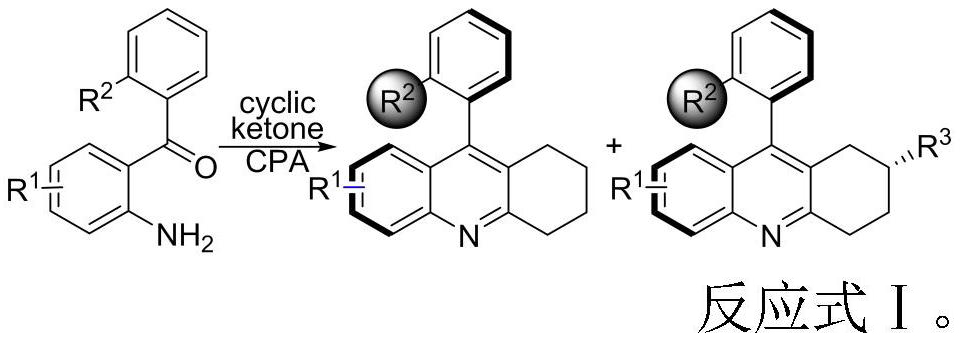
[0031] Sequentially add activated Molecular sieve powder (100mg), chiral phosphoric acid catalyst (10.0mg, 0.015mmol), 2-naphthylamine (5.7mg, 0.040mmol), (2-amino-4-chlorophenyl) (1-naphthyl) ketone ( 2-amino-4-chlorophenyl)(naphthalen-1-yl)methanone (0.10mmol), carbon tetrachloride (0.2mL), chloroform (0.8mL) and cyclohexanone (0.30mmol), with tetrafluoro thread seal cap. The reaction mixture was placed in a preheated 80°C oil bath for 4 days, and thin layer chromatography showed that the starting material had disappeared. The reaction process is shown in Reaction Formula II, and the catalyst structure is shown in Formula 1.
[0032] The reaction mixture was directly separated by silica gel column chromatography, using ethyl acetate:petroleum ether (30:1 to 10:1) as the mobile phase, followed by thin layer chromatography, and the product was collected. 26.4 mg of a white solid were obt...
Embodiment 2
[0035] Optimization and selection of embodiment 2 reaction conditions
[0036] Under the same reaction conditions as in Example 1, the different additives added have a significant impact on product and reaction rate, as shown in table 1, it is the impact on reaction and product when using different reaction solvents and additives,
[0037] The selection and output effect of additives in the reaction of table 1
[0038]
[0039] As shown in Table 1: The addition of molecular sieves can improve the enantioselectivity and reaction rate, and 2-naphthylamine can accelerate the reaction and slightly increase the enantioselectivity. The solvent is CCl 4 The reaction rate is faster, but the enantioselectivity becomes worse, while using CHCl 3 and CCl 4 A mixed solvent can reduce the reaction temperature, accelerate the reaction rate, and also improve the enantioselectivity of the compound reaction.
Embodiment 3
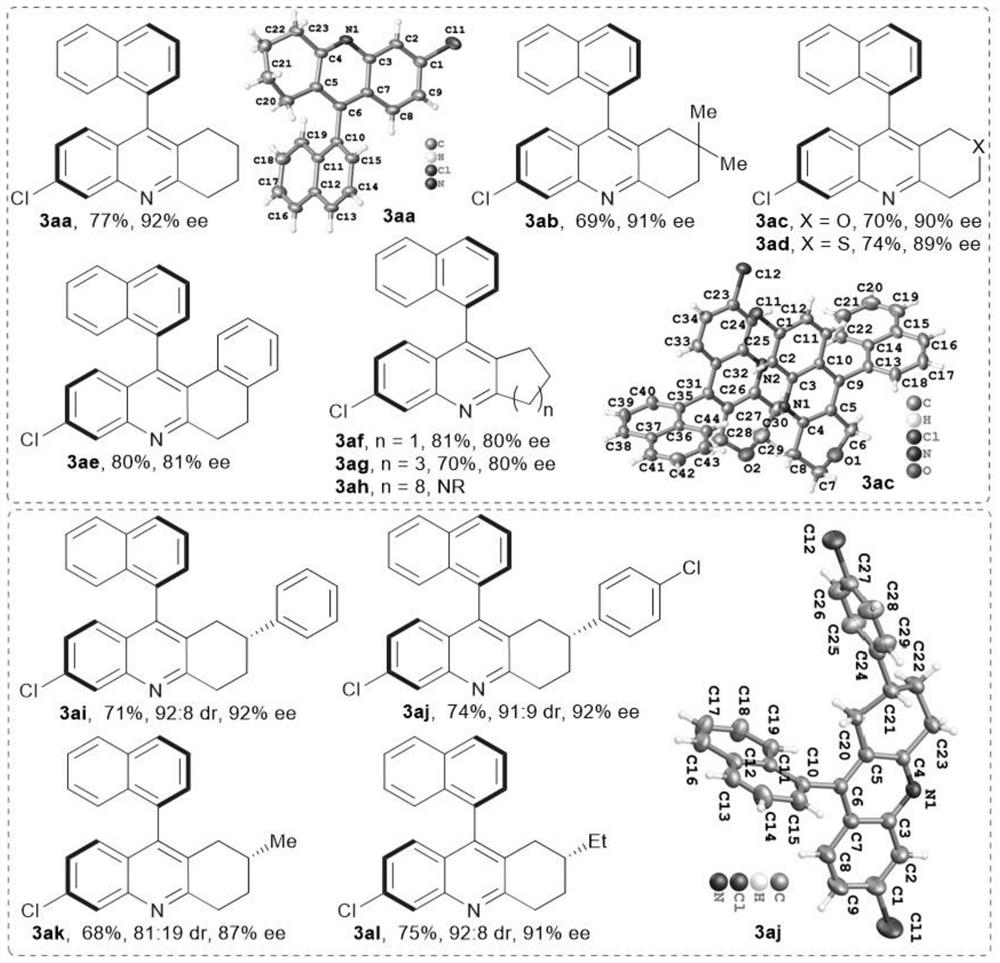
[0040] The selection of embodiment 3 substrate and corresponding product
[0041] 1, as figure 1 The alicyclic ketones shown as reaction substrates are reacted according to Reaction Formula III, (2-amino-4-chlorophenyl)(1-naphthyl)methanone 1a (28.1mg, 0.10mmol), alicyclic Ketone 2a-l (0.30mmol), chiral phosphoric acid (15mol%), 2-naphthylamine (40mol%), Molecular sieves (100mg) were added to CHCl 3 (0.8mL) and CCl 4 (0.2mL) in a mixed solvent at 80°C in an oil bath to seal the reaction. The 3aa-3ah reaction time is 4 days, and the 3ai-3al reaction time is 5 days. The above yields are isolated yields. The enantiomeric excess (ee value) and diastereomeric ratio (dr) of the product were separated and analyzed by HPLC chiral stationary phase method.
[0042]
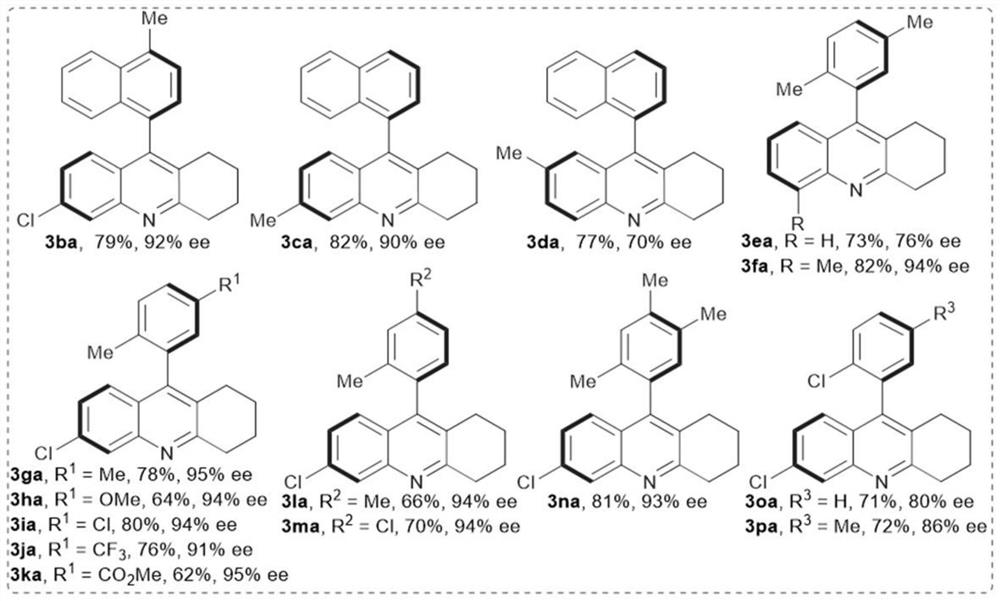
[0043] 2, as figure 2 The shown 2-aminoaryl ketone compound is used as the reaction substrate, and the reaction is carried out according to the reaction formula IV
[0044]
[0045] Reaction conditions: 2-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com