Method for determining content of six ginsenoside ingredients of folium ginseng
A technology of ginsenoside and determination method, which can be applied in the directions of measurement device, material separation, analysis material, etc., and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
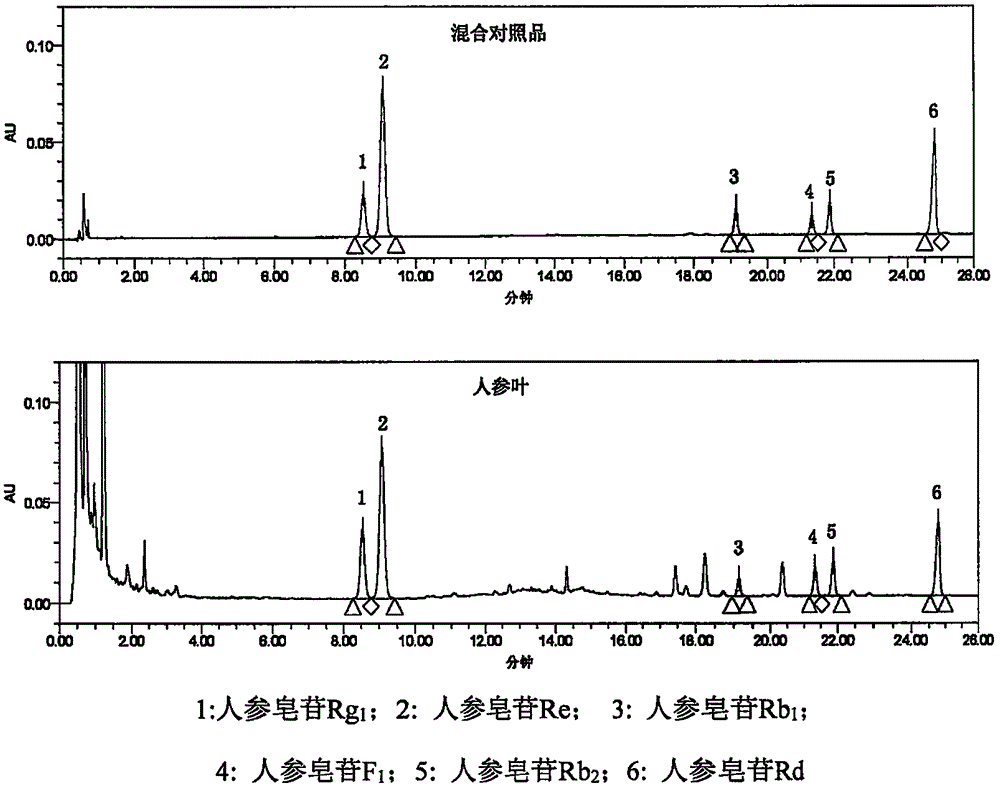
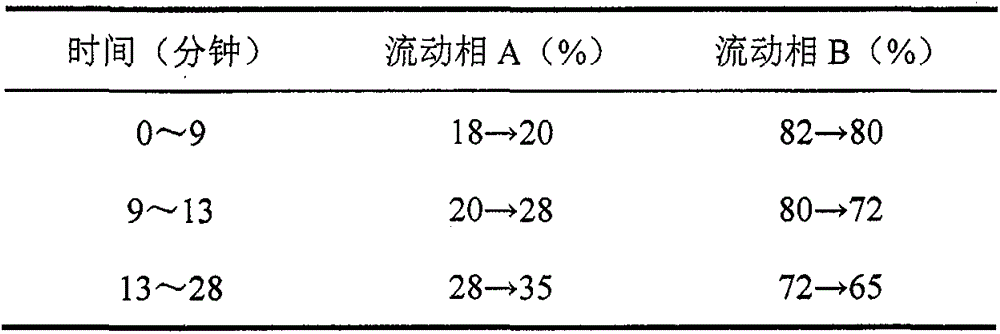
[0087] Chromatographic conditions and system suitability test The chromatographic column uses octadecylsilane bonded silica gel as a micro-column; acetonitrile is used as mobile phase A, water is used as mobile phase B, and gradient elution is carried out. The elution gradient is: 0 ~9 minutes, the mobile phase A gradually changes from 18% to 20%; 9~13 minutes, the mobile phase A gradually changes from 20% to 28%; 13~28 minutes, the mobile phase A gradually changes from 28% to 35%; the detection wavelength is 203nm; flow rate 0.4ml / min;
[0088] Preparation of mixed reference solution
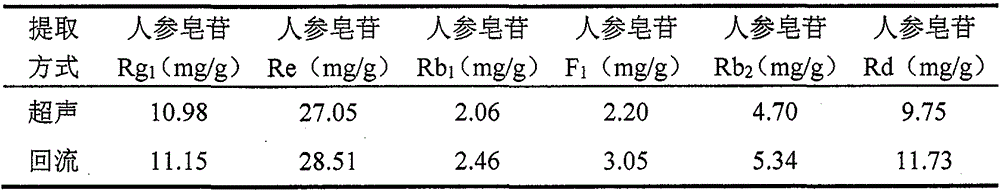
[0089] Weigh ginsenoside Rg 1 Reference substance, ginsenoside Re reference substance, ginsenoside Rb 1 Reference substance, ginsenoside Rb 2 Appropriate amount of reference substance, 20(S)-ginsenoside F1 reference substance, ginsenoside Rd reference substance, accurately weighed, and made by adding methanol. Each 1ml contains 100μg of ginsenoside Rg1, 500μg of ginsenoside Re, and ginsenosi...
Embodiment 2
[0093] Chromatographic conditions and system suitability test The chromatographic column uses octadecylsilane bonded silica gel as a micro-column; acetonitrile is used as mobile phase A, water is used as mobile phase B, and gradient elution is carried out. The elution gradient is: 0 ~9 minutes, the mobile phase A gradually changes from 18% to 20%; 9 to 13 minutes, the mobile phase A gradually changes from 20% to 28%; 13 to 28 minutes, the mobile phase A gradually changes from 28% to 35%; the detection wavelength is 203nm; flow rate 0.4ml / min;
[0094] Preparation of mixed reference solution
[0095] Weigh ginsenoside Rg 1 Reference substance, ginsenoside Re reference substance, ginsenoside Rb 1 Reference substance, ginsenoside Rb 2 Appropriate amount of reference substance, 20(S)-ginsenoside F1 reference substance, and ginsenoside Rd reference substance, accurately weighed, and made by adding methanol, each 1ml contains ginsenoside Rg 1 100μg, Ginsenoside Re500μg, Ginsenos...
Embodiment 3
[0099] Chromatographic conditions and system suitability test The chromatographic column uses octadecylsilane bonded silica gel as a micro-channel column; acetonitrile is used as mobile phase A, water is used as mobile phase B, and gradient elution is carried out. The elution gradient is: 0 ~9 minutes, mobile phase A gradually changes from 18% to 20%; 9 ~ 13 minutes, mobile phase A gradually changes from 20% to 28%; 13 ~ 28 minutes, mobile phase A gradually changes from 28% to 35%; detection wavelength is 203nm; flow rate 0.4ml / min;
[0100] Preparation of mixed reference solution
[0101] Weigh ginsenoside Rg 1 Reference substance, ginsenoside Re reference substance, ginsenoside Rb 1 Reference substance, ginsenoside Rb 2 Appropriate amount of reference substance, 20(S)-ginsenoside F1 reference substance, and ginsenoside Rd reference substance, accurately weighed, and made by adding methanol, each 1ml contains ginsenoside Rg 1 100μg, Ginsenoside Re500μg, Ginsenoside Rb 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com