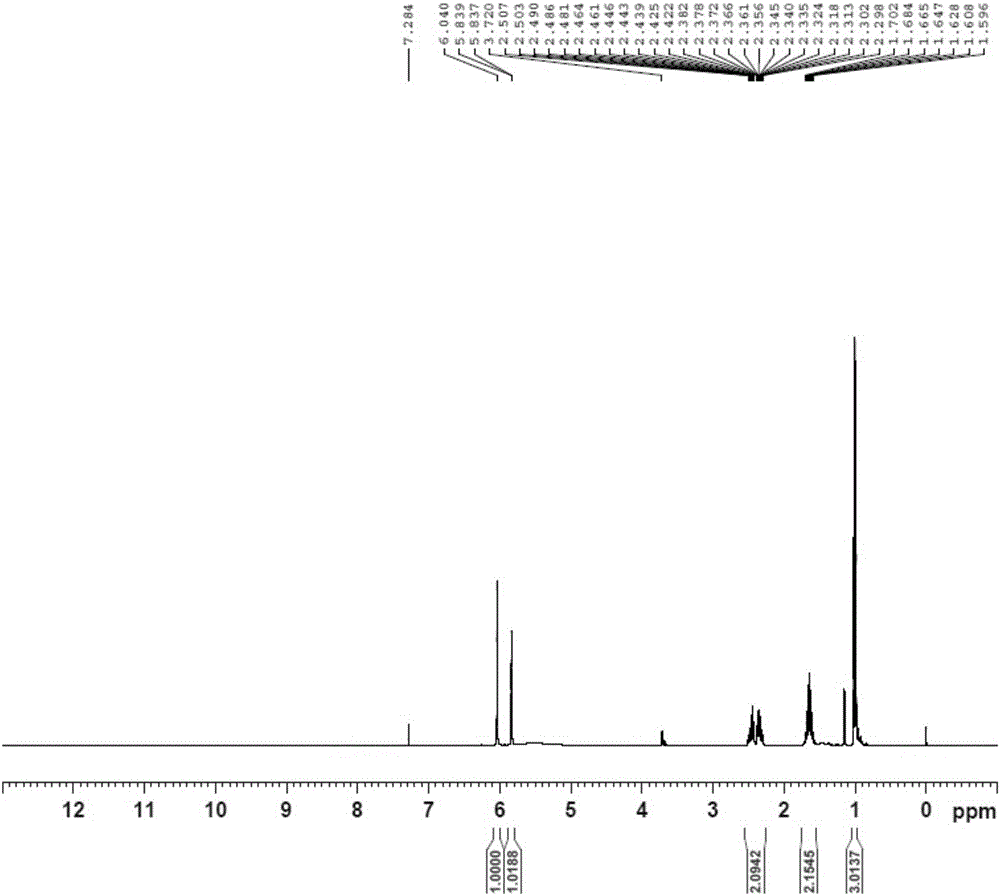
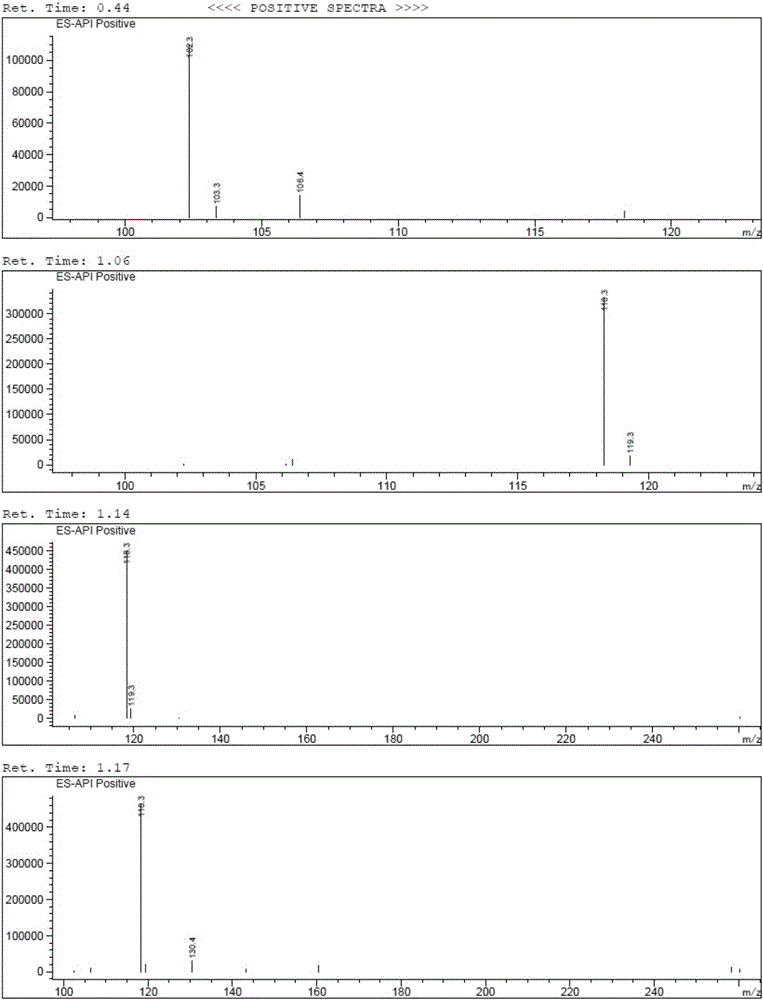
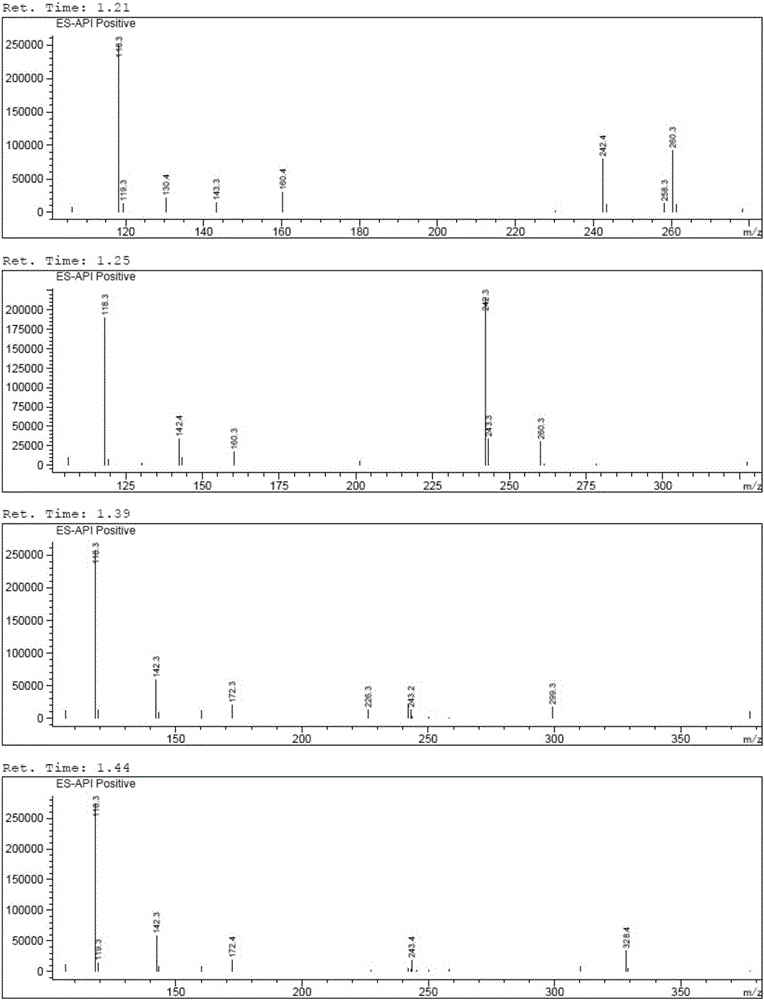
Brivaracetam and preparation method of intermediate thereof
An intermediate and volumetric technology, applied in the field of medicine, can solve the problems of poor industrial feasibility and high production cost, and achieve the effects of reduced preparation cost, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 The preparation technology of Bu Waracetam of the present invention
[0061] synthetic route:
[0062]
[0063] The specific process includes the following steps:
[0064] (1) Preparation of 5-hydroxyl-4-propylfuran-2(5H)-ketone (i.e. B-I)
[0065] Add 50% glyoxylic acid aqueous solution (60g, 0.411mol), n-heptane (100ml), water (30ml) in the 500ml there-necked flask with mechanical stirring function, cool down to below 10 ℃, add dropwise morphine (35.7g , 0.411mol), the dropwise temperature was raised to 25°C, reacted for 2h, added n-valeraldehyde (35.3g, 0.411mol), the temperature was raised to 45°C, after the reaction for 5h, the temperature was lowered to below 10°C, and concentrated hydrochloric acid ( 50ml), reacted for 3h, separated, separated the n-heptane phase, and washed the water phase (30ml×3) with n-heptane, the water phase was extracted with isopropyl ether (60ml×3), and the isopropyl ether phase was extracted with a concentration of Wa...
Embodiment 2
[0080] Embodiment 2 The preparation technology of Bu Waracetam of the present invention
[0081] (1) Preparation of B-I
[0082] Add 50% glyoxylic acid aqueous solution (60g, 0.411mol), n-heptane (100ml), water (30ml) in the 500ml there-necked flask with mechanical stirring function, cool down to below 10 ℃, add dropwise morphine (53.55g , 0.6155mol), the dropwise temperature was raised to 25°C, reacted for 2h, added n-valeraldehyde (52.95g, 0.6165mol), the temperature was raised to 60°C after the addition, after the reaction for 3h, the temperature was lowered to below 10°C, and concentrated hydrochloric acid ( 50ml), heated to 40°C for 1h, separated, separated the n-heptane phase, and washed the water phase with n-heptane (30ml×3), the water phase was extracted with isopropyl ether (60ml×3), and the isopropyl ether phase Wash with 30% sodium bicarbonate aqueous solution (30ml×2), saturated brine (30ml×1), dry over anhydrous sodium sulfate, concentrate the isopropyl ether ph...
Embodiment 3
[0097] Embodiment 3 The preparation technology of Bu Waracetam of the present invention
[0098] (1) Preparation of B-I
[0099] Add 50% glyoxylic acid aqueous solution (60g, 0.411mol), n-heptane (100ml), water (30ml) in the 500ml there-necked flask with mechanical stirring function, cool down to below 10 ℃, add dropwise morphine (42.84g , 0.4932mol), the dropwise temperature was raised to 25°C, reacted for 2h, added n-valeraldehyde (70.6g, 0.822mol), the temperature was raised to 45°C after the addition, after the reaction for 7h, the temperature was lowered to below 10°C, and concentrated hydrochloric acid ( 50ml), heated to 40°C for 1h, separated, separated the n-heptane phase, and washed the water phase with n-heptane (30ml×3), the water phase was extracted with isopropyl ether (60ml×3), and the isopropyl ether phase Wash with 30% sodium bicarbonate aqueous solution (30ml×2), saturated brine (30ml×1), dry over anhydrous sodium sulfate, concentrate the isopropyl ether phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com