A kind of preparation method of 3-aminomethyltetrahydrofuran
A technology for cyanotetrahydrofuran and tetrahydrofuran, applied in directions such as organic chemistry, can solve problems such as difficulty in synthesizing tetrahydrofuran compounds, unfavorable industrial production, expensive ruthenium catalysts, etc., and achieves the effects of low production cost, little environmental pollution, and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
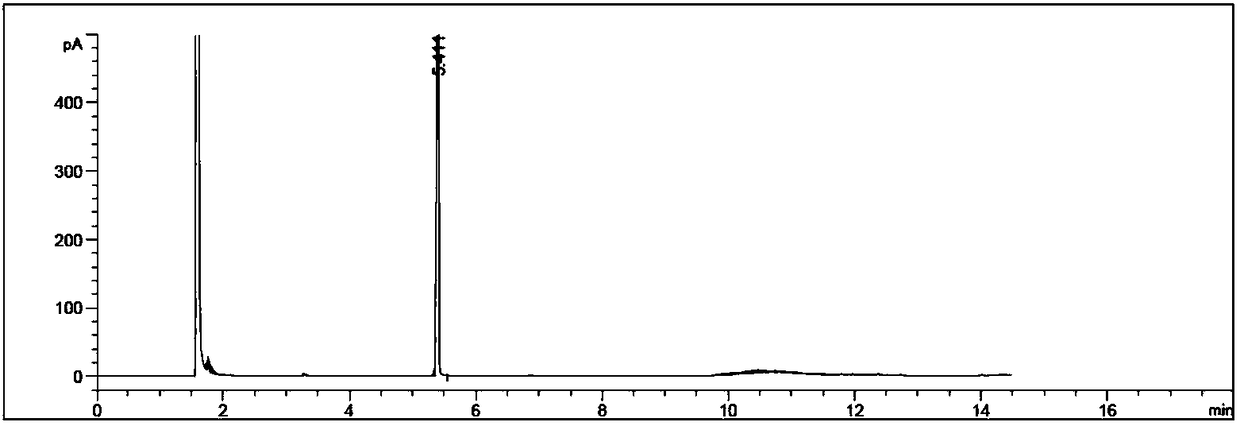
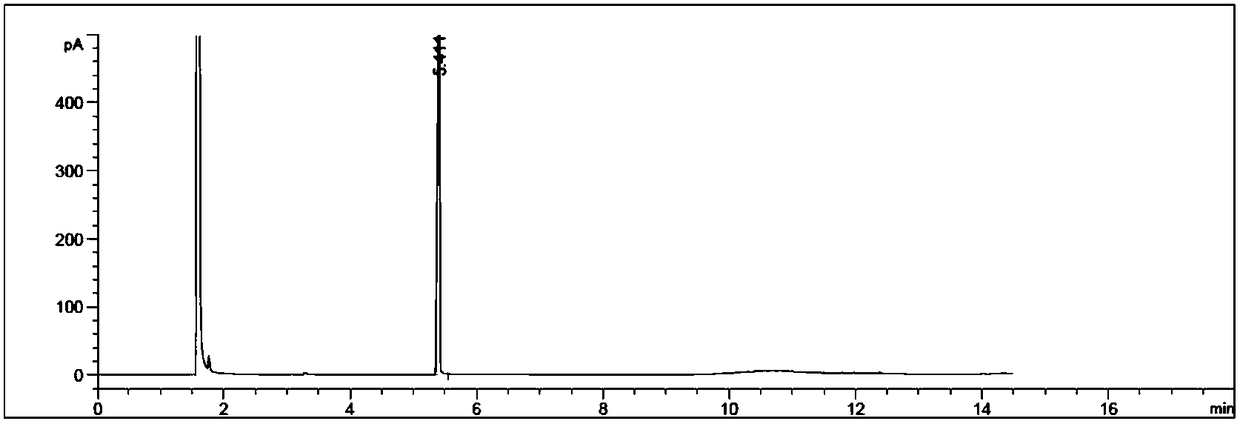
Image
Examples
Embodiment 1
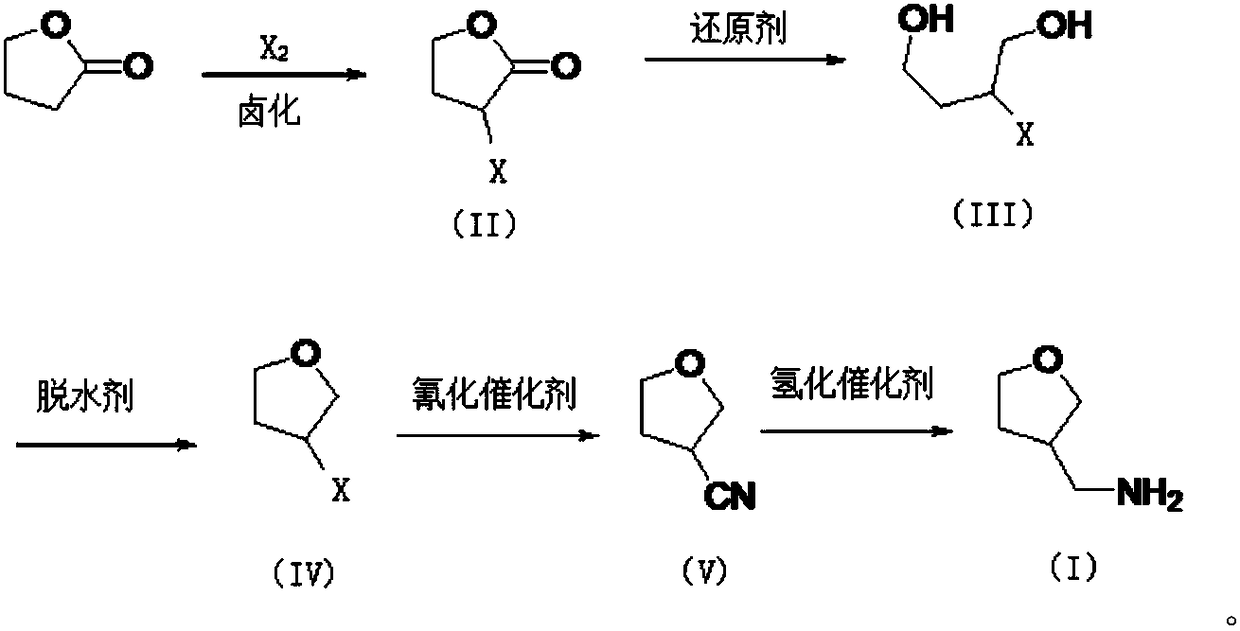
[0022] Embodiment 1: (1) halogenation reaction
[0023]
[0024] Weigh 500g (5.8mol) of γ-butyrolactone into a 1000ml four-necked bottle, equip with condensation and tail gas absorption device, stir and heat to 120°C, start to introduce chlorine gas, the temperature gradually rises to 140°C, and maintain the temperature at 140~ 160°C, continuously feed 494g (6.96mol) of chlorine gas for 12h, then directly rectify the reaction solution under reduced pressure to recover unreacted γ-butyrolactone, and then collect 3-chloro-γ-butyrolactone stably, as Colorless oil, namely 3-chloro-γ-butyrolactone; purity 95%, yield 70%.
[0025]
[0026] (2) Reduction reaction
[0027] Weigh 120g (1.0mol) of 3-chloro-γ-butyrolactone and place it in a 1000ml four-necked bottle, add 500ml of anhydrous methanol to dissolve, add 22.68g (0.6mol) of sodium borohydride in batches at 25°C, and Stir the reaction at 25°C for 4-6 hours, gas phase detection until the reaction is complete, evaporate th...
Embodiment 2
[0041] (1) Halogenation reaction
[0042]Weigh 500g (5.8mol) of γ-butyrolactone into a 1000ml four-necked bottle, equip with condensation and tail gas absorption device, stir and heat to 120°C, start to introduce chlorine gas, the temperature gradually rises to 140°C, and maintain the temperature at 140~ 160°C, continuously feed 494g (6.96mol) of chlorine gas for 12h, then directly rectify the reaction solution under reduced pressure to recover unreacted γ-butyrolactone, and then collect 3-chloro-γ-butyrolactone stably, as Colorless oil, namely 3-chloro-γ-butyrolactone; purity 95%, yield 70%.
[0043] (2) Reduction reaction
[0044] Weigh 120g (1.0mol) of 3-chloro-γ-butyrolactone into a 1000ml four-necked bottle, add 500ml of anhydrous tetrahydrofuran to dissolve, add 37.95g (1.0mol) of lithium tetrahydrohydride in batches at 20°C, and Stir the reaction at 20°C for 3h, the gas phase detects that the reaction is complete, cool down to 0°C, and slowly add 200ml of water under ...
Embodiment 3
[0054] (1) Halogenation reaction
[0055] Weigh 500g (5.8mol) of γ-butyrolactone into a 1000ml four-necked bottle, equip with condensation and tail gas absorption device, stir and heat to 120°C, start to introduce chlorine gas, the temperature gradually rises to 140°C, and maintain the temperature at 140~ 160°C, continuously feed 494g (6.96mol) of chlorine gas for 12h, then directly rectify the reaction solution under reduced pressure to recover unreacted γ-butyrolactone, and then collect 3-chloro-γ-butyrolactone stably, as Colorless oil, namely 3-chloro-γ-butyrolactone; purity 95%, yield 70%.
[0056] (2) Reduction reaction
[0057] Weigh 120g (1.0mol) of 3-chloro-γ-butyrolactone and place it in a 1000ml four-necked bottle, add 500ml of anhydrous methanol to dissolve, add 22.68g (0.6mol) of sodium borohydride in batches at 25°C, and Stir the reaction at 25°C for 6 hours. When the gas phase detects that the reaction is complete, distill methanol off under reduced pressure, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com