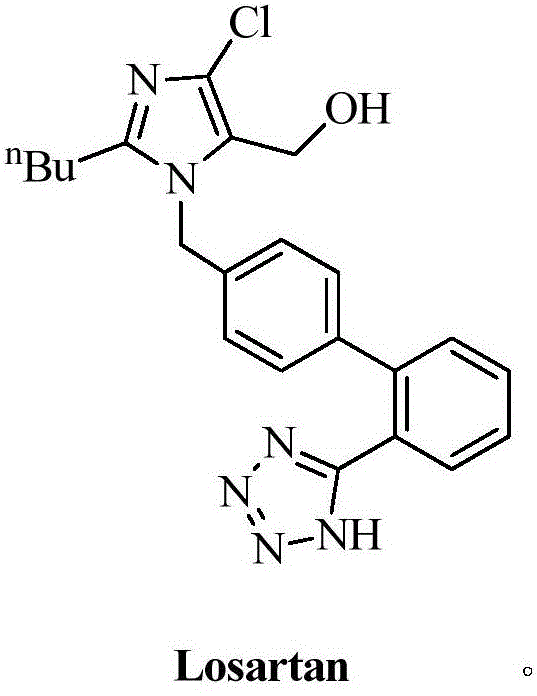
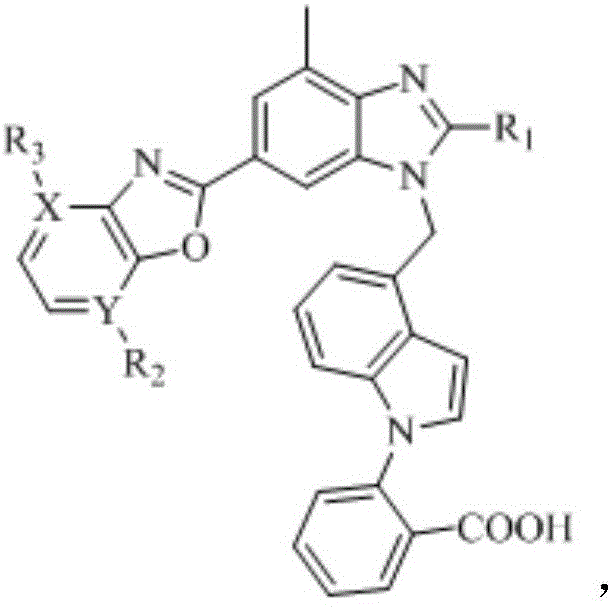
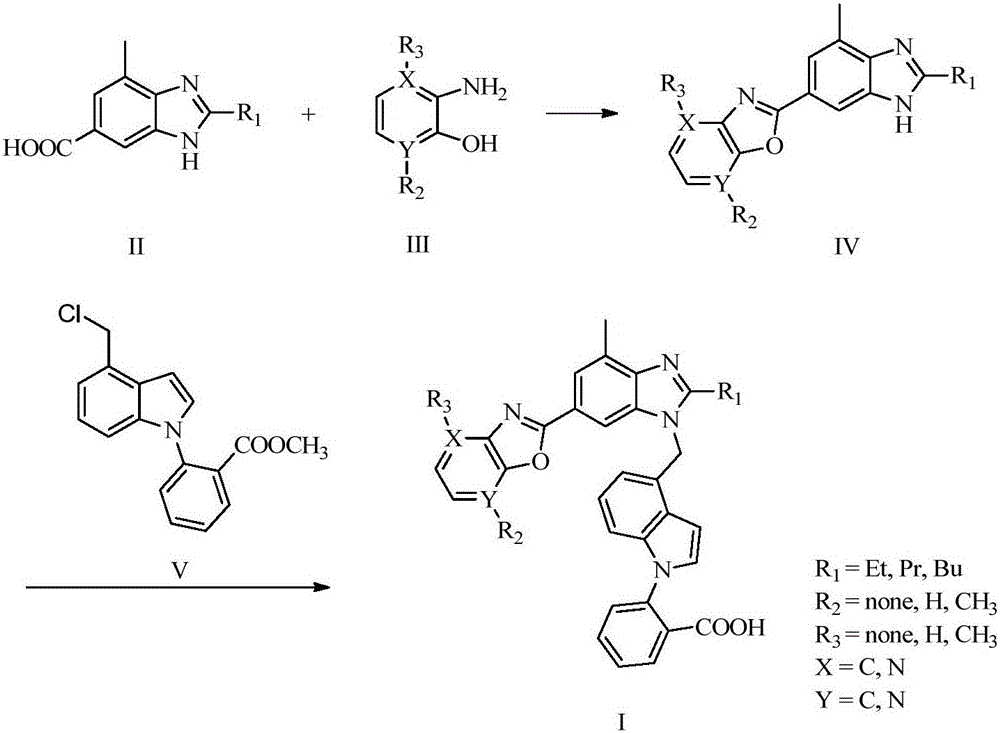
6'-substituted benzimidazole-4-substituted methylindole derivative and preparation and application thereof
A technology of methyl indole derivatives and benzimidazoles, which is applied in the field of antagonists and their preparation and application, and can solve problems such as metabolic instability, AII agonism, and restriction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 2-{4-{[2-Propyl-4-methyl-6-(benzo[d]oxazol-2-yl)-1H-benzo[d]imidazol-1-yl]methyl}- The preparation method of 1H-indol-1-yl}benzoic acid (compound Ib):
[0056] Step 1: Synthesis of 4-methyl-2-propyl-6-(4-benzoxazol-2-yl)benzimidazole IVb:
[0057]
[0058] Compound IIc (534 mg, 2.45 mmol) was slowly added to polyphosphoric acid (15 mL) heated to 80°C, stirred and heated to 150°C. Compound IIIb (320mg, 2.94mmol) was slowly added to the former polyphosphoric acid solution several times. After the addition was complete, the reaction was stirred at 150°C for 12h. After the reaction is complete, pour the reaction solution into 100 mL of ice water, and adjust the pH to 8-10 with concentrated ammonia water, a large amount of precipitate will appear, filter, and wash the filter cake three times with 5% ethanol solution. After the filter cake was dried, the resulting filter cake was purified by column chromatography to obtain about 357 mg of light yellow solid Compound IVb ...
Embodiment 2
[0063] 2-{4-{[2-Ethyl-4-methyl-6-(benzo[d]oxazol-2-yl)-1H-benzo[d]imidazol-1-yl]methyl}- The preparation method of 1H-indol-1-yl}benzoic acid (compound Ia):
[0064] The experimental procedure was as described in Example 1, and the yield was 56.3%. 1 H NMR (400MHz, DMSO) δ12.93(s,1H),8.17(s,1H),7.93(m,2H),7.80-7.69(m,3H),7.64-7.48(m,3H),7.39- 7.37(m,2H),7.04-7.00(m,2H),6.73(d,J=3.2Hz,1H),6.33(d,J=5.8Hz,1H),5.95(s,2H),2.91(q ,J=7.5Hz,2H),2.69(s,3H),1.31(t,J=7.5Hz,3H). 13 C NMR(101MHz,DMSO)δ167.66,163.89,159.24,150.64,144.97,142.21,137.66,137.20,136.04,132.94,131.09,130.55,130.55,129.54,128.89,128.89,128.48,126.45,125.44,125.16,122.52,121.51 ,120.12,119.82,116.61,111.14,109.79,107.92,101.14,45.18,20.87,16.93,12.04. MS (ESI) m / z: 527.2 [M+H] + .
Embodiment 3
[0066]2-{4-{[2-Butyl-4-methyl-6-(benzo[d]oxazol-2-yl)-1H-benzo[d]imidazol-1-yl]methyl}- The preparation method of 1H-indol-1-yl}benzoic acid (compound Ic):
[0067] The experimental procedure was as described in Example 1, and the yield was 48.6%. 1 H NMR(400MHz,DMSO)δ12.90(s,1H),8.15(s,1H),7.98-7.88(m,2H),7.79-7.68(m,3H),7.62-7.52(m,3H), 7.43-7.33(m,2H),7.09-6.95(m,2H),6.74(d,J=3.3Hz,1H),6.34(dd,J=5.6,2.3Hz,1H),5.95(s,2H) ,2.89(t,2H),2.67(s,3H),1.76-1.68(m,2H),1.41-1.31(m,2H),0.85(t,J=7.3Hz,3H). 13 C NMR(101MHz,DMSO)δ167.56,163.88,158.34,150.63,145.02,142.20,137.77,137.20,135.91,133.17,131.18,130.53,130.34,129.48,128.96,128.96,128.52,126.48,125.44,125.16,122.54,121.52 ,120.07,119.82,116.67,111.14,109.75,107.95,101.22,45.23,29.57,27.09,22.38,16.92,14.15.MS(ESI)m / z:555.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com