Antibody testing method for efficacy of a classical swine fever genetically engineered subunit vaccine
A subunit vaccine and genetic engineering technology, applied in the field of veterinary biological products, can solve the problems of long testing period, shortening testing period, high cost of feeding and testing, and achieve short testing period, saving testing cost and high biological safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: A kind of swine fever genetic engineering subunit vaccine (293T-E2) potency testing method
[0019] Include the following steps:
[0020] (1) Get 20 healthy susceptible rabbits (rabbits whose ELISA antibody is negative for classical swine fever virus) as test objects, and randomly divide them into 4 groups according to body weight and sex, with 5 rabbits in each group; 3 groups are immune groups, 1 group is the control group;
[0021] (2) Inject the rabbits in the immunized group. Three groups are injected with 0.1ml, 0.2ml, and 0.5ml of the CSF genetically engineered subunit vaccine (293T-E2) respectively, while the rabbits in the control group do not inject any substance; inject the vaccine The rabbits in the control group and the rabbits in the control group were raised according to the normal feeding method;
[0022] (3) After 21 days of step (2) injection of the vaccine, the rabbits in the immunization group and the control group were all subjected ...
Embodiment 2
[0027] Example 2: Correlation detection between antibody detection and virus attack protection:
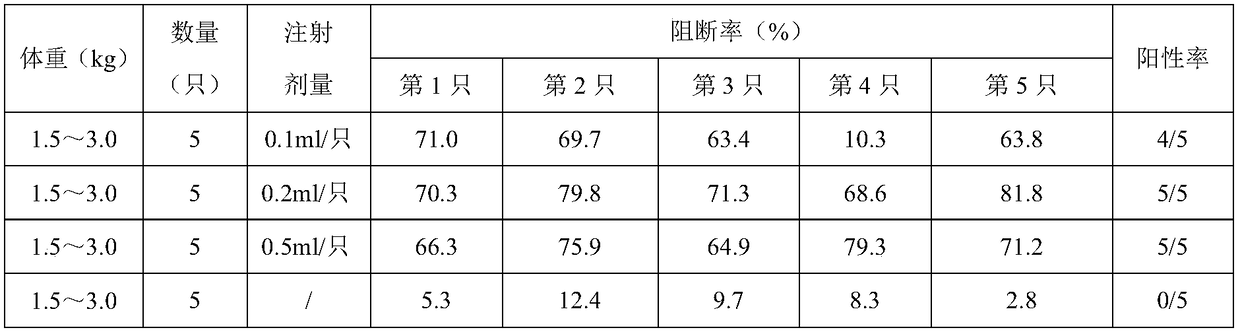
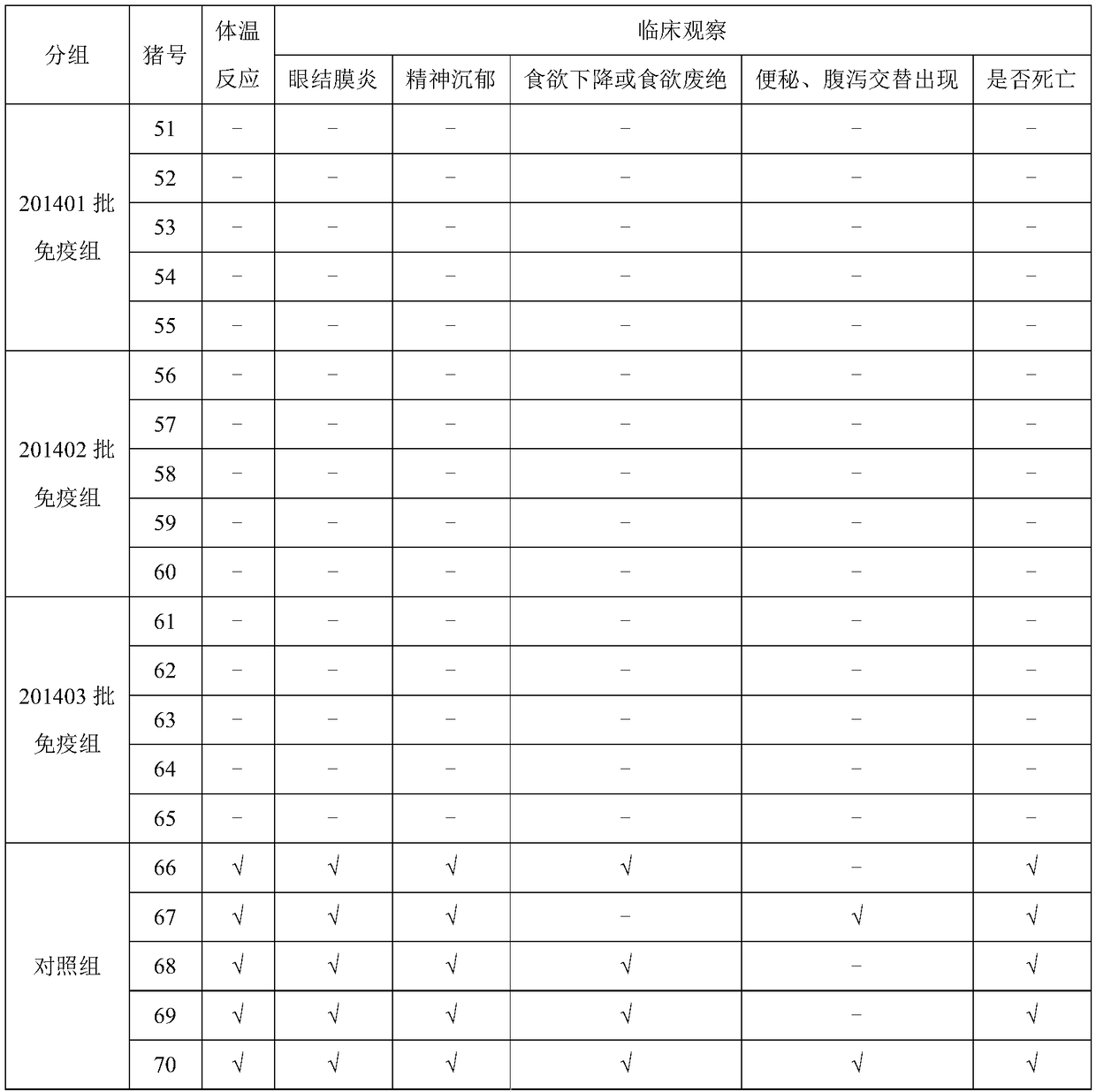
[0028] In embodiment 1, after the rabbits of the immunization group and the control group carried out heart blood collection, each rabbit carried out ear vein injection of live vaccine for swine fever (spleen leaching source), 1.0ml / only; The body temperature was measured once a day for the first two days of the injection, and every 6 hours after the injection, for a total of 72 hours; when the temperature change of the rabbit before and after the injection did not exceed 1°C, it was determined that the protective effect had been achieved. The results are shown in Table 2:
[0029] Table 2: Attack Protection
[0030] group
[0031] It can be seen from Table 2 that after the rabbits are injected with the CSF genetically engineered subunit vaccine (293T-E2), they are then challenged with the CSF virus (the attenuated strain of CSF in the CSF splenogenous live vaccine), wh...
Embodiment 3
[0032] Embodiment 3: Application of the test method of the present invention and comparison with target animal test
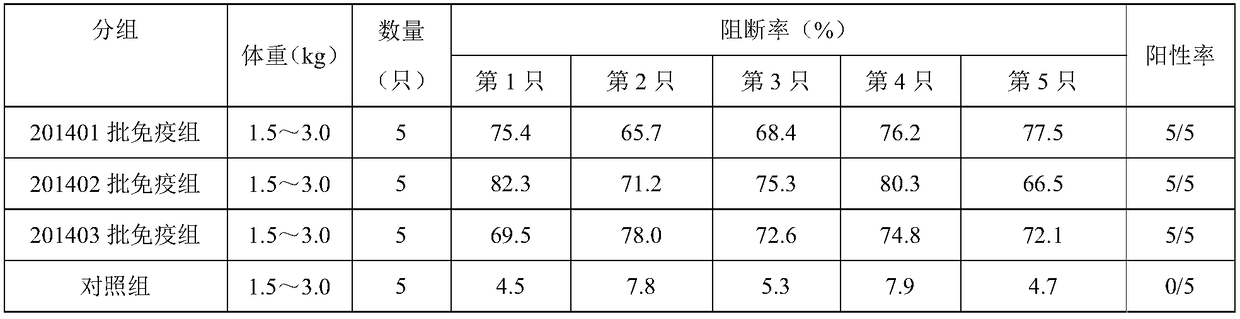
[0033] Take 20 healthy and susceptible rabbits weighing 1.5-3.0kg (the ELISA antibodies of pestivirus are all negative) and divide them into 4 groups at random, with 5 rabbits in each group, and inject 201401, 201402, and 201403 batches of pigs subcutaneously in each neck of groups 1-3 Pestilence gene engineering subunit vaccine (293T-E2), 0.2ml / only, the 4th group is the control group. On the 21st day after immunization, blood was collected from the heart of all rabbits, centrifuged at 7000r / min for 10 minutes, and the supernatant was drawn to detect the ELSIA antibody to classical swine fever; if the antibody was positive, it proved that the genetic engineering subunit vaccine (293T-E2) had achieved protective effect. The results are shown in Table 3:
[0034] Table 3: Antibody detection
[0035]
[0036] Note: Blocking rate ≥ 40% is positive (+); 30% < ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com