Optical isomer of 1,4-benzothiazepine-1-oxide derivative, and pharmaceutical composition prepared using same
A technology of optical isomers and pharmaceutical compositions, applied in the field of optical isomers of 1,4-benzothiazepine*-1-oxide derivatives and pharmaceutical compositions using it, can solve unavoidable proarrhythmic effect and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Compound 4-[3-(4-benzylpiperidin-1-yl)propionyl]-7-methoxy-2,3,4,5-tetrahydro-1,4- represented by formula [Ia] Benzothiazepines - Manufacture of 1-oxide
[0131] In the reaction vessel, 30.0 g of 4-[3-(4-benzylpiperidin-1-yl)propionyl]-7-methoxy-2,3, 4,5-tetrahydro-1,4-benzothiazepine hydrochloride, adding solvent chloroform (CHCl 3 ) 800ml, stirred at room temperature to dissolve it. Next, the reaction container was immersed in an ice-water bath, and cooled until the temperature in the container reached 0 to 1°C. Here, while paying attention not to increase the reaction temperature, 14.0 g of m-chloroperbenzoic acid (mCPBA) was dissolved in chloroform (CHCl 3 ) The solution of 600ml was slowly dripped in with the dripping time of 110 minutes. After completion of the dropping, the mixture was stirred at 0°C to 1°C for about 20 minutes.
[0132] Next, 4.14g Na 2 SO 3 200ml of H 2 O solution was added dropwise at 0°C to 5°C over 1 minute, and stirred at 0°C to...
Embodiment 2
[0139] The first component and the second component of the optical isomers of the compound represented by the formula [IV] of the present invention are produced by separating and separately collecting the compound represented by the formula [Ia] produced in Example 1 under the following conditions .
[0140] Column: CHIRALPAK AD-H (manufactured by Daicel Corporation)
[0141] Size: inner diameter 0.46cm x length 25cm
[0142] Mobile phase: MeOH / MeCN / DEA=90 / 10 / 0.1(v / v)
[0143] Flow rate: 1.0mL / min
[0144] Temperature: 40°C
[0145] Detection wavelength: 245nm
[0146] Injection volume: 10μL
[0147] MeOH means methanol, MeCN means acetonitrile, and DEA means diethylamine.
[0148] Meanwhile, as an instrument, use:
[0149] Pump: LC-20AD (manufactured by Shimadzu Corporation)
[0150] Detector: SPD-20A (manufactured by Shimadzu Corporation)
[0151] Autosampler: SIL-20A (manufactured by Shimadzu Corporation)
[0152] From 10 g of the compound represented by the formula...
Embodiment 3
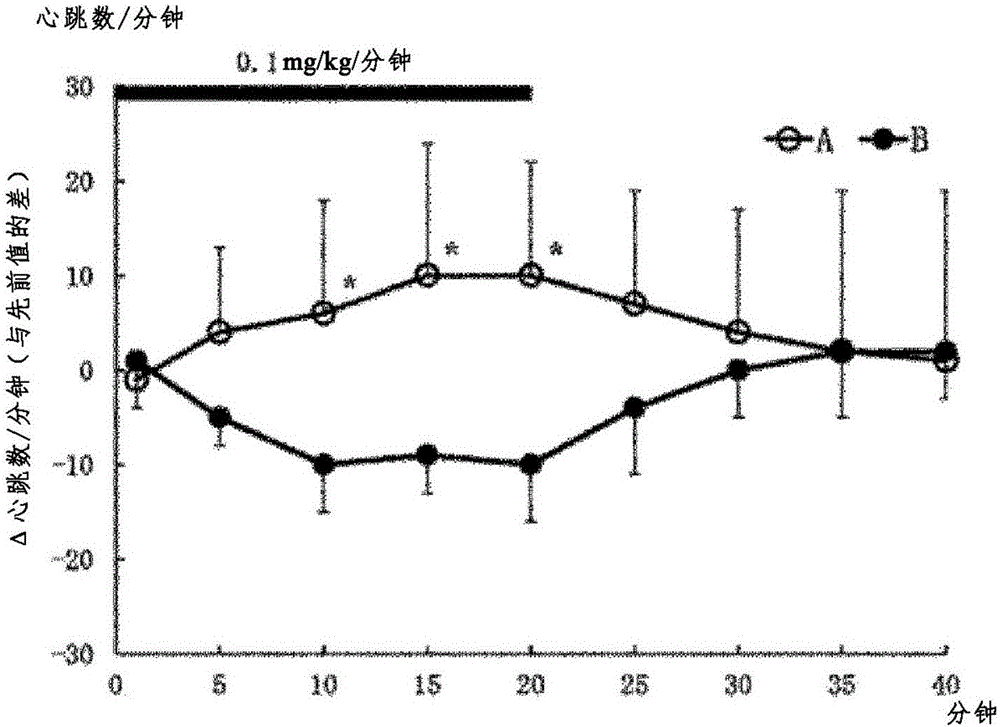
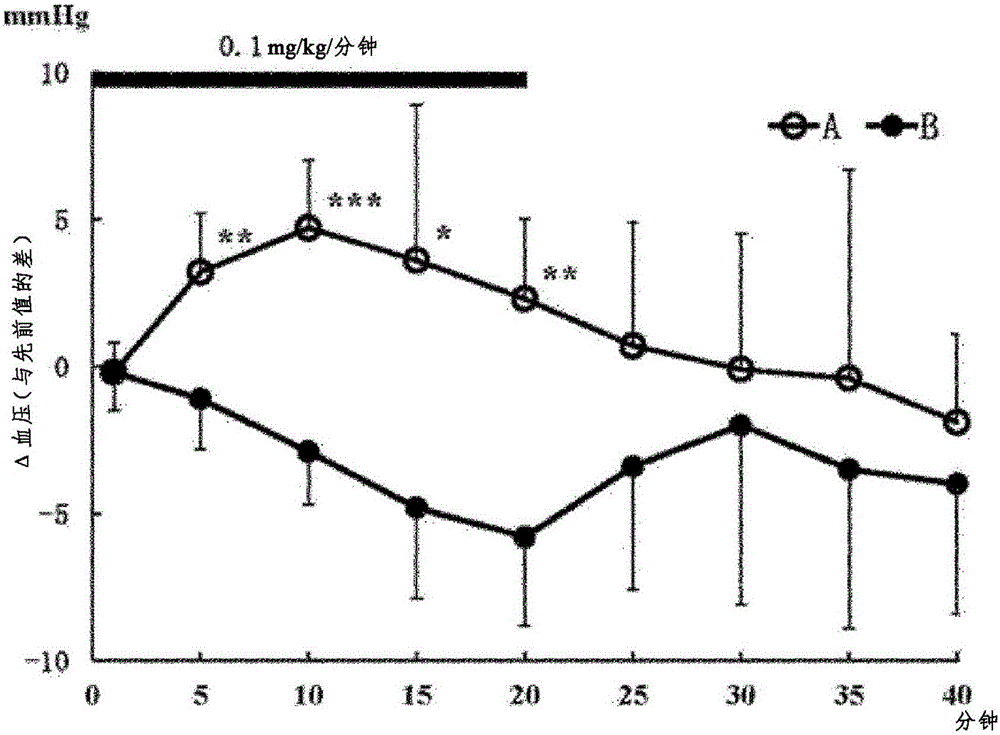
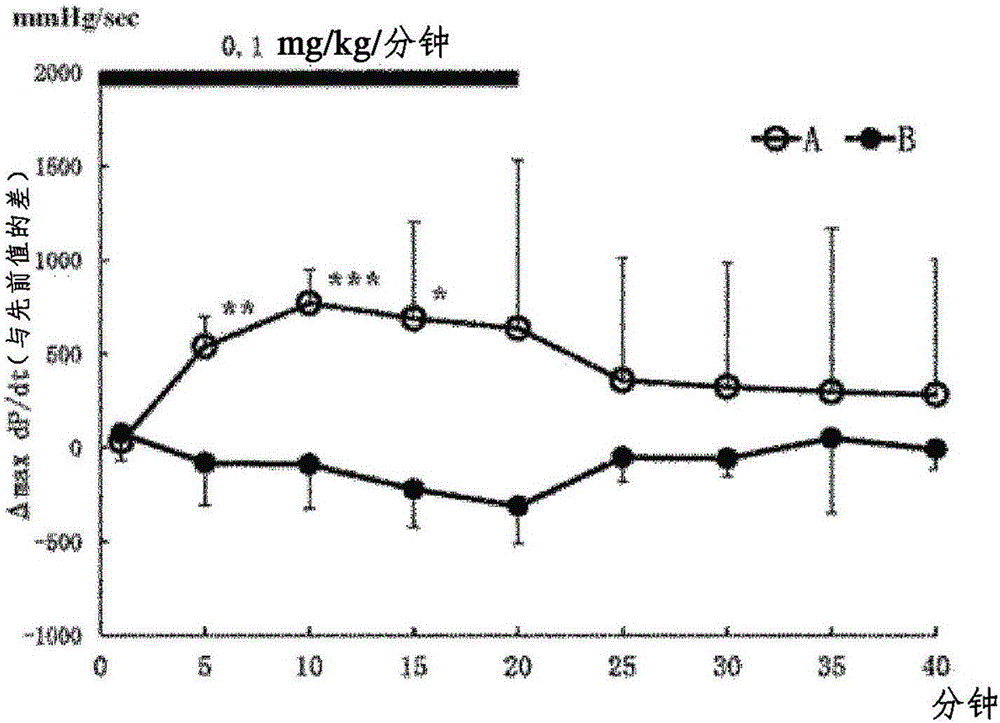
[0155] Determination of heartbeat, blood pressure, left ventricular systolic function (max dP / dt), left ventricular diastolic function (min dP / dt)
[0156] Test method: In this test, using rats under anesthesia, the hydrochloride of the optical isomer 1st component (A) and the optical isomer 2nd component (B) were administered continuously intravenously, respectively. Effects of medication on the circulatory system. Each group was performed with n=5. The first component (A) or the second component (B) was continuously administered at 0.1 mg / kg / min for 20 minutes, and heart rate, blood pressure, max dP / dt, and min dP / dt were measured. Measure the parameters of 0 minutes, 1 minute, 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, 30 minutes, 35 minutes, and 40 minutes of administration, so as to compare with the 0 minute value (previous value (control value)) The difference indicates. The measured values are expressed as mean ± SD.
[0157] Test result: the resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com