Catalyst used for synthesis of hexanediamine
A catalyst, a technology for hexamethylene diamine, which is applied in the field of catalysts for synthesizing hexamethylene diamine, can solve the problems of high toxicity of raw material adiponitrile, dependence on imports, hidden safety hazards, etc., so as to reduce one-time investment and production cost, and reduce operation difficulty. , the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
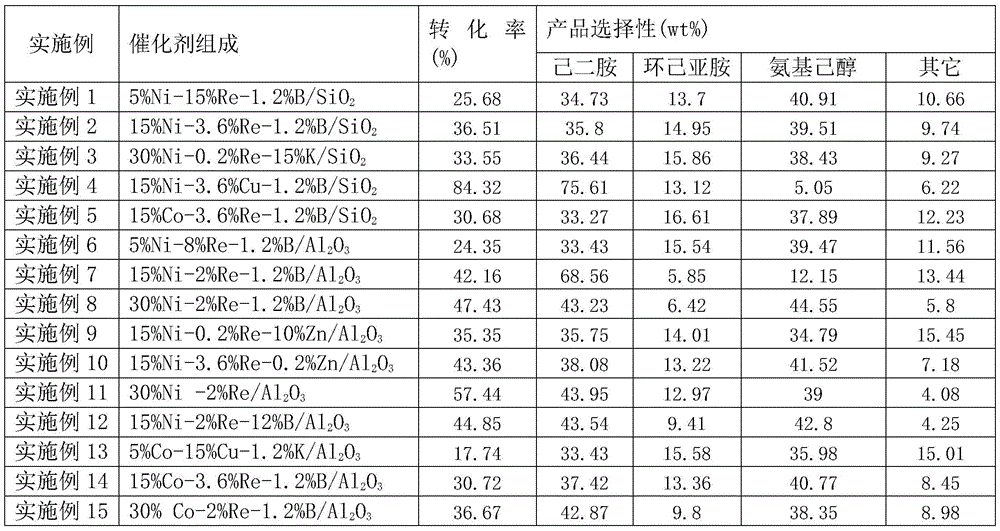
[0026]5%Ni-15%Re-1.2%B / SiO 2 Catalyst preparation and application
[0027] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Put it in a quartz tube, and bake it at 500°C for 5 hours under an inert atmosphere. 2.477 g Ni(NO 3 ) 2 ·6H 2 O, 2.161 g NH 4 ReO 4 and 0.686 g H 3 BO 3 Dissolve in 12ml deionized water. The above SiO was impregnated with half of this aqueous solution 2 The carrier was air-dried, then dried at 120°C for 4 hours, and then calcined at 500°C for 4 hours. Then, the above SiO was impregnated for the second time with the remaining half of the aqueous solution 2 The carrier is then air-dried, dried at 120°C for 4 hours, and calcined at 500°C for 4 hours. Before the catalyst is used, in a hydrogen flow at 375°C (atmospheric pressure, 2000h -1 ) to reduce for 4 hours. When the temperature in the reactor is naturally lowered to 160°C, the pressure is increased to 8MPa. After the system is stable, the NH 3 / 1,6-Hexanediol = 5 (molar ratio) l...
Embodiment 2
[0029] 15%Ni-3.6%Re-1.2%B / SiO 2 Catalyst preparation and application
[0030] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Put it in a quartz tube, and bake it at 500°C for 5 hours under an inert atmosphere. 7.432 g Ni(NO 3 ) 2 ·6H 2 O, 0.518 g NH 4 ReO 4 and 0.686 g H 3 BO 3 Dissolve in 12ml deionized water. Refer to Example 1 for the remaining preparation steps and catalyst evaluation scheme. The reaction results are shown in Table 1.
Embodiment 3
[0032] 30%Ni-0.2%Re-15%K / SiO 2 Catalyst preparation and application
[0033] Weigh 10 g carrier SiO 2 (20-40 mesh), the carrier SiO 2 Put it in a quartz tube, and bake it at 500°C for 5 hours under an inert atmosphere. 14.864 g Ni(NO 3 ) 2 6H2O, 0.029 g NH 4 ReO 4 and 3.879 grams of KNO 3 Dissolve in 12ml deionized water. Refer to Example 1 for the remaining preparation steps and catalyst evaluation scheme. The reaction results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com