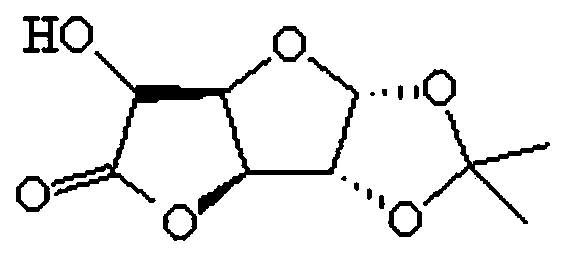
A method for preparing 1,2-oxygen-isopropylidene-alpha-d-6,3-glucuronolactone
A technology of -alpha-d-6 and isopropylidene, applied in the field of sugar chemistry, can solve the problems of multiple reaction steps, complex process, high risk, etc., and achieve the effect of high selectivity, simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The invention provides a method for preparing 1,2-oxo-isopropylidene-alpha-D-6,3-glucuronolactone, comprising the following steps:
[0031] (1) Propylene protection reaction: Add 10 grams of glucose and 40 grams of hydrogen-type cation exchange resin to 500 milliliters of acetone, heat at 55°C and stir for 6 hours, cool to 25°C, add 200 milliliters of water, and continue the reaction at room temperature After 5 hours, filter off the resin, and recover acetone under vacuum at 50°C to obtain 1,2-oxo-isopropylidene-alpha-D-glucose solution;
[0032] (2), uronic acid oxidation reaction: 0.1 g of 10% platinum carbon is added to 1,2-oxo-isopropylidene-alpha-D-glucose solution, and 10% sodium hydroxide solution is added dropwise at 55°C for control The pH value is 9, the pressure is maintained at 0.01MPa by feeding oxygen, and the catalyst is recovered by filtration for 6 hours. The filtrate is vacuum concentrated at 50°C to a concentration of 35%, and the sodium ion is remove...
Embodiment 2
[0035] The invention provides a method for preparing 1,2-oxo-isopropylidene-alpha-D-6,3-glucuronolactone, comprising the following steps:
[0036] (1) Propylene protection reaction: Add 10 grams of glucose and 15 grams of hydrogen-type cation exchange resin to 250 milliliters of acetone, heat at 30°C and stir for 12 hours, cool to 25°C, add 50 milliliters of water, and continue the reaction at room temperature After 2 hours, the resin was filtered off, and acetone was recovered under vacuum at 50°C to obtain 1,2-oxo-isopropylidene-alpha-D-glucose solution;
[0037] (2), uronic acid oxidation reaction: 0.3 grams of 5% platinum carbon is added to 1,2-oxo-isopropylidene-alpha-D-glucose solution, and 10% sodium hydroxide solution is added dropwise at 35°C for control PH value 10, feed oxygen to maintain pressure 0.01MPa, stir vigorously for 3 hours, filter and recover the catalyst, concentrate the filtrate in vacuum at 50°C to 20% concentration, pass through cation exchange resin ...
Embodiment 3
[0040] The invention provides a method for preparing 1,2-oxo-isopropylidene-alpha-D-6,3-glucuronolactone, comprising the following steps:
[0041] (1) Propylene protection reaction: Add 10 grams of glucose and 30 grams of hydrogen-type cation exchange resin to 400 milliliters of acetone, heat at 40°C and stir for 9 hours, cool to 25°C, add 100 milliliters of water, and continue the reaction at room temperature After 3 hours, the resin was filtered off, and acetone was recovered under vacuum at 50°C to obtain 1,2-oxo-isopropylidene-alpha-D-glucose solution;
[0042](2), uronic acid oxidation reaction: 0.2 g of 5% platinum carbon is added to 1,2-oxo-isopropylidene-alpha-D-glucose solution, and 10% sodium hydroxide solution is added dropwise at 30°C for control PH value 11, feed oxygen to maintain pressure 0.01MPa, stir vigorously for 2 hours, filter and recover the catalyst, concentrate the filtrate in vacuum at 50°C to 30% concentration, pass through cation exchange resin to re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com