TAL effector-mediated DNA modification
一种效应子、特异性的技术,应用在使用转录激活因子样效应子序列领域,能够解决有效方法难以实现等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Example 1 - Determining the code for TAL effector-DNA recognition
[0212] To determine whether there is a one-to-one linear correspondence between consecutive nucleotides in the RVD and TAL target sites, the TAL effector RVD sequences were used to scan the predicted promoter regions of each of the 10 TAL effector known target genes (i.e. 1,000 bp in front of the translation start site is shown) for alignments that minimize entropy in RVD nucleotide associations (arbitrary). The following formula is used to quantify entropy, where R is the RVD group of effectors, D is the group of 4 nucleotides (A, C, G, T), and fi,j represents the observed i-th RVD associated with j-th Nucleotide frequency:
[0213]
[0214] Various low-entropy sites exist in each promoter. For the effector AvrBs3, however, only one mapped to the 54bp upa20 promoter fragment previously identified as necessary and sufficient for activation and which is consistent with the UPA box common to genes ...
Embodiment 2
[0239] Example 2 - TALEN can work in yeast
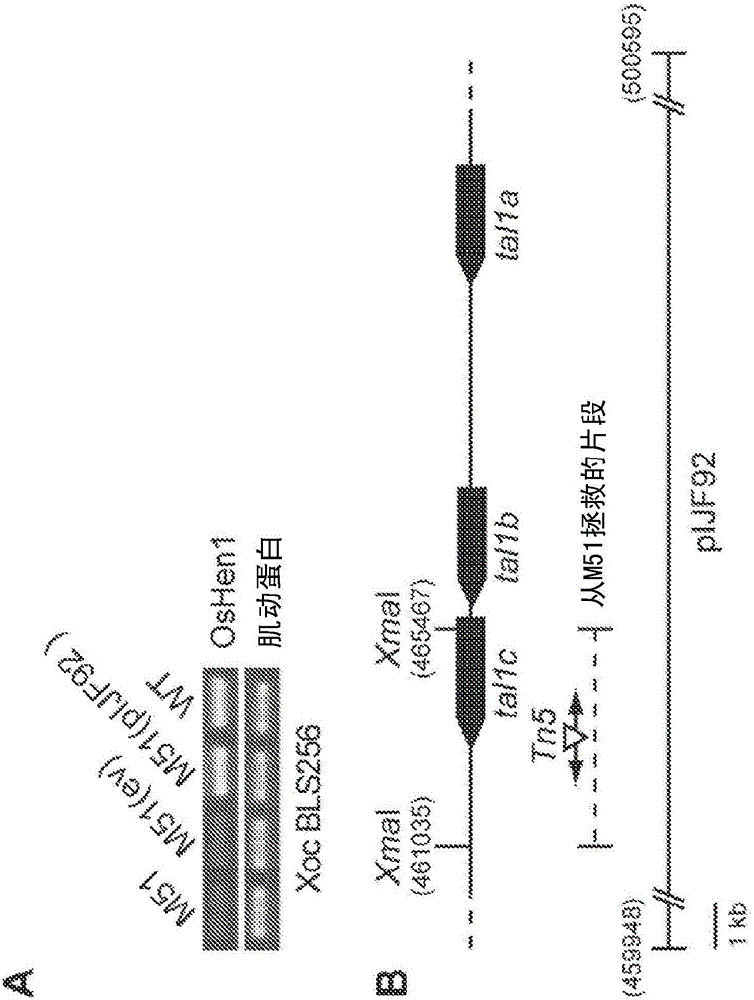
[0240] Plasmid construction: The protein coding sequence of TAL effector AvrBs3 was obtained by digesting the plasmid with BamHI. The DNA segment primarily encoding the repeat domain was cleaved with SphI. The amino acid sequence of AvrBs3 can be found in GENBANK accession number P14727 and SEQ ID NO: 12 ( image 3 ), the nucleotide sequence is found in Accession No. X16130 and SEQ ID NO: 13 ( Figure 4 )middle. exist Figure 4 , BamHI and SphI sites are shown in bold and underlined. The AvrBs3 BamHI and SphI fragments were cloned into the nuclease expression vector pDW1789_TAL (Fig. 5), adjacent to the sequence encoding the FokI nuclease domain. To clone the AvrBs3 target site into the target reporter plasmid, two complementary DNA oligomers containing two AvrBs3 recognition sites arranged in reverse and an 18 bp spacer sequence between them were synthesized at the 5' and 3' ends, respectively. Has BglII and SpeI prominentl...
Embodiment 3
[0245] Example 3 - Modular Assembly of TAL Effector Repeats for Custom TALENs
[0246] Complementary oligonucleotides of 102 base pairs corresponding to each of the four individual TAL effector repeats, each assigned a different nucleotide, were synthesized, annealed, and either individually or in 2 and 3 of all permutations Combinatorial cloning of repeat sequences into high-copy bacterial cloning vectors was performed using standard restriction digestion and ligation techniques to generate 4 single repeat modules, 16 double repeat modules, and 64 triple repeat modules (e.g. Figure 11 shown). The desired TAL effector coding sequence was assembled by introducing the appropriate modular sequence into a Gateway-ready high-copy bacterial cloning vector comprising a truncated version of the tal1c gene that lacks the central repeat region except for the final characteristic half repeat. For example, an 18-repeat TAL effector coding sequence can be assembled by sequentially int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com