Preparation method and application of modified enhanced type targeting immune cell mass
A cell and interleukin technology, applied in the fields of cell biology, immunology, biology, and tumor treatment, can solve problems such as side effects and unsatisfactory treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1. Preparation of lentivirus expressing human PD-1 fragment
[0059] 1. Construction of human PD-1 fragment lentivirus
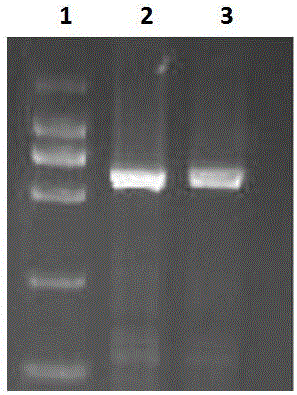
[0060] Synthesize the double-stranded DNA molecule shown in SEQ ID NO: 4 of the sequence listing, link it into a commercial lentiviral vector, take the pWPXL vector system as an example, including but not limited to the pWPXL vector, and construct the recombinant plasmid pWPXL-PD -1- IRES-IL-2, identified by PCR, the results are as follows figure 1 As shown, lane 1 is the DL2000 Marker, lane 2 is the identification result of PD-1, and lane 3 is the identification result of IL-2.
[0061] Virus packaging is accomplished by the following routine:
[0062] a. The recombinant expression plasmid and the helper plasmid were co-transfected into HEK293 cells by the calcium phosphate method. Inoculate HEK293 cells in DMEM medium containing 10% fetal bovine serum, culture at 37ºC and 5% CO2 until logarithmic growth phase, collect cells, inoculat...
Embodiment 2
[0070] Example 2, Detection of PD-1 after recombinant virus infected CIK cells
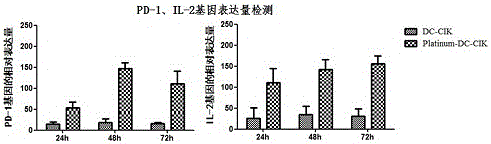
[0071] In order to fully illustrate the beneficial effects of the present invention, the present invention also performs real-time PCR detection of PD-1 and IL-2 transcription levels in CIK cells infected with pWPXLd-PD-1-IRES-IL-2, the steps are as follows :
[0072] Total RNA was extracted from cells infected with pWPXLd–PD-1-IRES-IL-2 for 4h, 8h, and 12h respectively. At the same time, CIK cells infected with pWPXLd were used as controls, and uninfected CIK cells were used as blank controls. Then it was reverse-transcribed into a cDNA template, and the designed specific primers were used to detect whether the mRNAs of PD-1 and IL-2 were transcribed, and GAPDH was used as an internal reference. The primer sequences for GAPDH are:
[0073] Upstream primer: 5'-ACCACAGTCCATGCCATCAC-3'
[0074] Downstream primer: 5'-TCCACCACCCCTGTTGCTGTA-3';
[0075] The primer sequence of PD-1 is:
[0076] Ups...
Embodiment 3
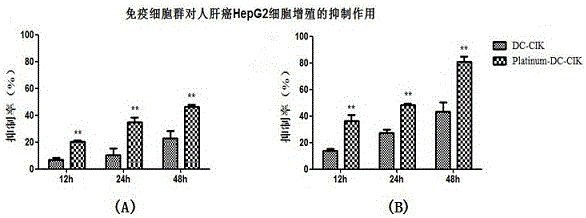
[0083] Example 3, Preparation of modified DC-CIK (Triumph-DC-CIK) cells
[0084] 8. Preparation of PBMC from Peripheral Blood Mononuclear Cells
[0085] Take fresh anticoagulated whole blood, EDTA (sodium citrate or heparin) anticoagulant can be used. Dilute whole blood with an equal volume of PBS or 0.9% NaCl. Add a certain volume of separation liquid into the centrifuge tube, spread the diluted blood sample above the liquid surface of the separation liquid, and keep the interface between the two liquid surfaces clear. Separation solution, anticoagulated undiluted whole blood, PBS (or normal saline) at a volume of 1:1:1, centrifuged at 2000rpmx25min, separated to obtain PBMC, then washed twice with Hanks solution, counted the number of cells under a microscope, and finally used serum-free The culture medium VIVO-15 adjusted the cell density to make 5x106 / ml cell suspension;
[0086] 9. Non-adherent vs. Adherent Cell Isolation
[0087] Transfer the cell suspension into a 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com