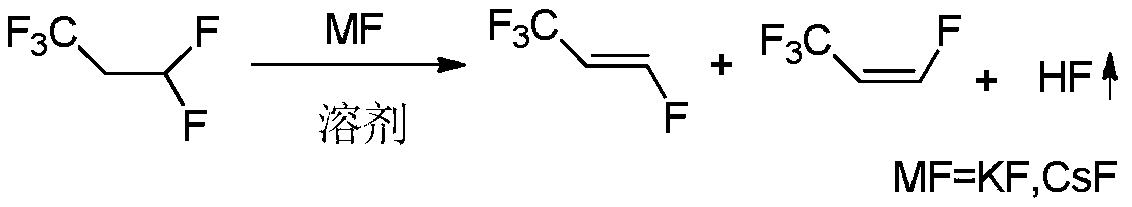
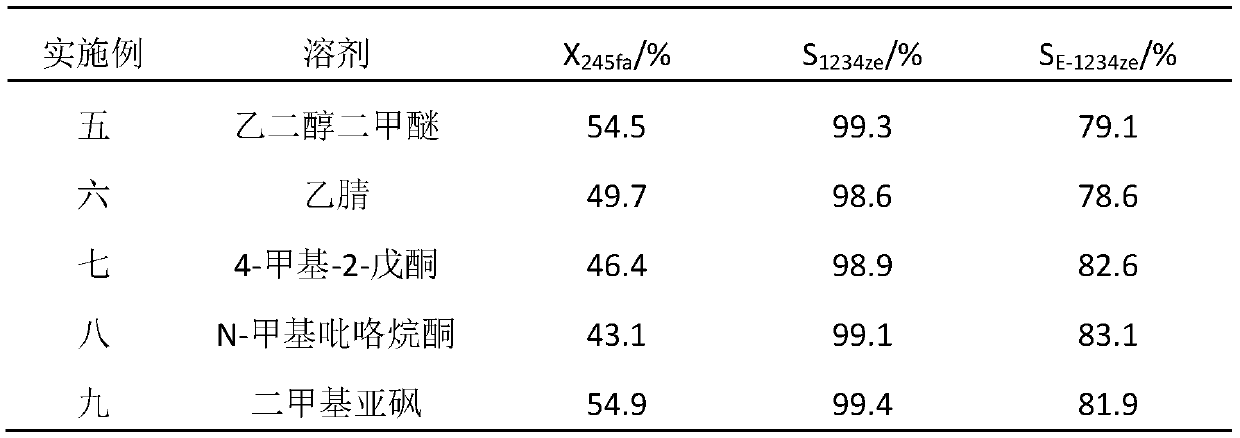
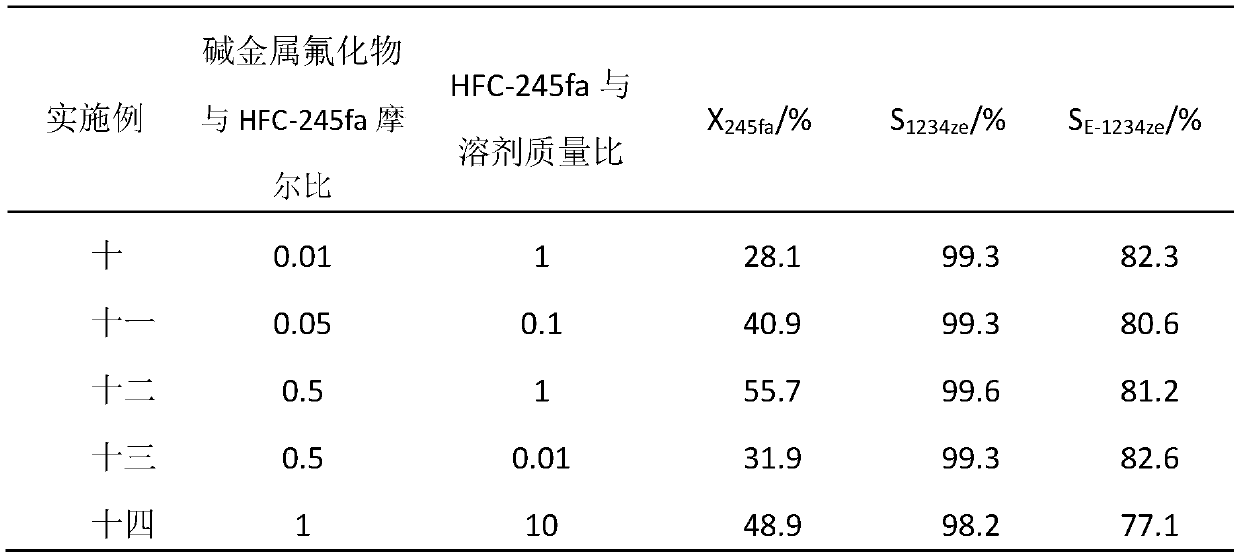
A method for synthesizing trans-1,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and pentafluoropropane, which is applied in the field of synthesizing trans-1,3,3,3-tetrafluoropropene, can solve the problems of unfriendly environment, troublesome post-processing, and difficult to recycle, and achieve an environment-friendly Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The reaction was carried out in a stirred 500mL stainless steel autoclave. Put 5.2g of highly active anhydrous potassium fluoride, 119g of N,N-dimethylformamide, and 119g of HFC-245fa into the reaction kettle in sequence, start stirring, raise the reaction temperature to 120°C, and keep the reaction temperature after 8 hours of reaction , from the gas phase port of the reactor, the reaction materials such as HFC-245fa, HFO-1234ze and HF with low boiling point are slowly exhausted. The conversion of -245fa was 55.9%, the overall selectivity of HFO-1234ze(E / Z) was 99.5%, and the selectivity of E-HFO-1234ze was 81.4%.
Embodiment 2
[0017] Add 119g of HFC-245fa to the reaction kettle after the reaction in Example 1, and continue to heat up to 120°C for reaction. Analysis of the reaction products showed that the conversion rate of HFC-245fa was 55.3%, the total selectivity of HFO-1234ze (E / Z) was 99.6%, and the selectivity of E-HFO-1234ze was 82.1%. The results of Comparative Example 1 show that the potassium fluoride and organic solvents after removal of HFC-245fa, HFO-1234ze and HF still have high reactivity and can continue to be used for the next batch of reactions.
Embodiment 3
[0019] The operation process of embodiment three is similar to embodiment one, and difference is to replace highly active anhydrous potassium fluoride with cesium fluoride, and reaction product analysis shows that the transformation efficiency of HFC-245fa is 60.5%, and HFO-1234ze (E / Z ) was 99.4% overall selectivity, wherein the selectivity of E-HFO-1234ze was 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com