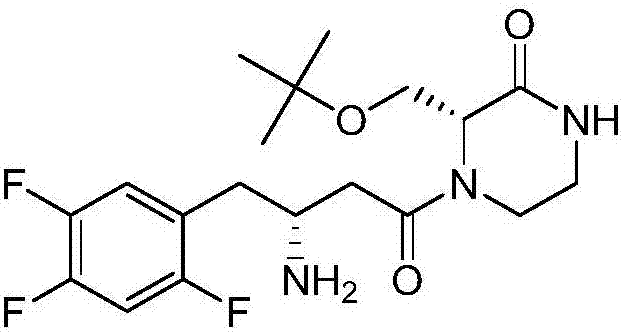
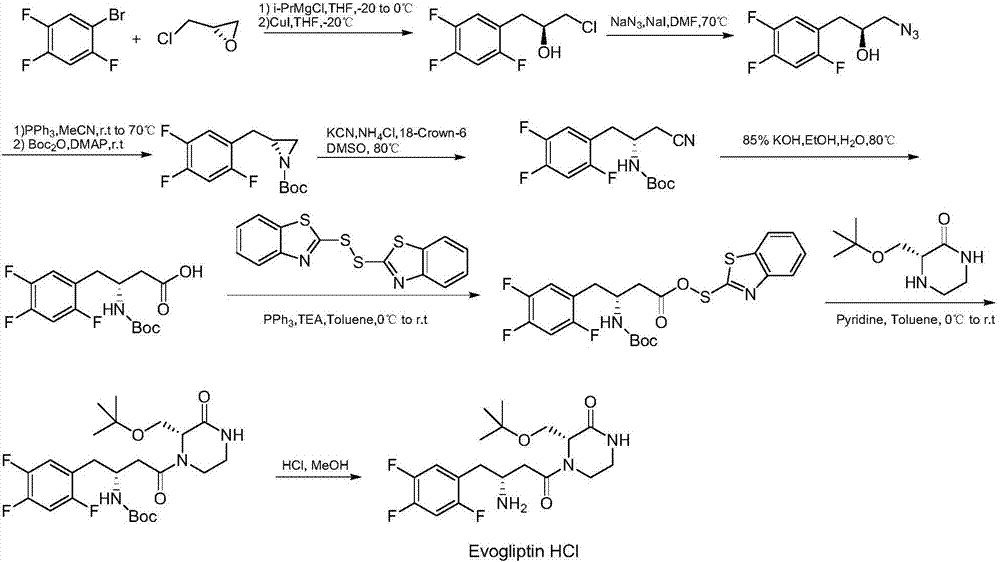
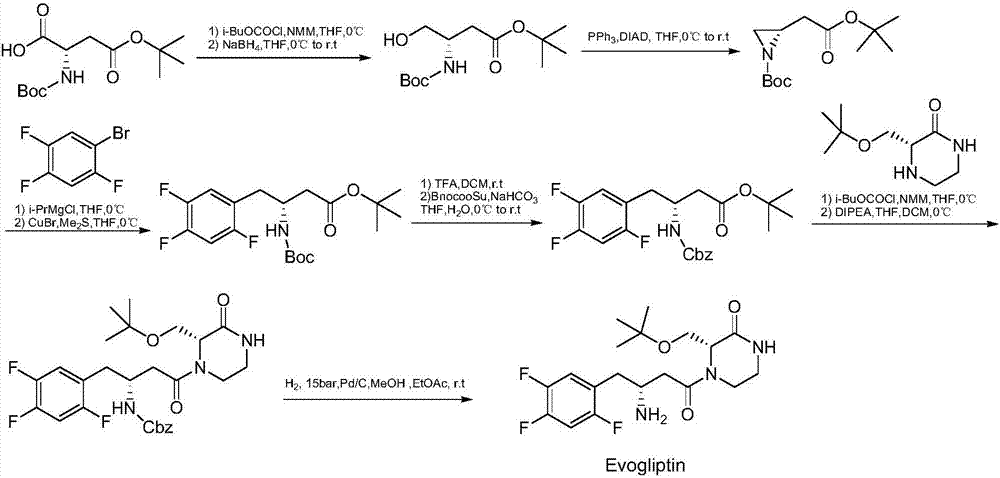
Preparation method of evogliptin
An equation and compound technology, applied in the field of organic chemical synthesis, can solve the problems of high cost, long synthetic route steps and high production cost, and achieve the effects of mild reaction conditions, low cost of raw materials and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 formula I compound
[0060] Add 2,4,5-trifluorophenylacetic acid (38 g, 0.2 mol) into 1200 ml of dry tetrahydrofuran, start stirring, slowly add CDI (31 g, 0.22 mol), slightly exothermic, after the addition, add The reaction mixture was heated to 50°C. Isopropyl malonate (32 g, 0.22 mol) was added to the reaction system in batches, the reaction temperature was maintained at 50°C, and the addition was completed in about 30 minutes. After the addition, the reaction was continued for 4 hours. Concentrate the reaction solution under reduced pressure, pour the residue into a mixture of 200ml of water and 300ml of dichloroethane, adjust the pH to 2 with 0.1N dilute hydrochloric acid, separate the organic layer, and wash with 200ml of water and 200°C saturated brine in sequence The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated to obtain intermediate 1 (compound of formula I) as a white solid: 58.4 g, with a...
Embodiment 2
[0063] The preparation of embodiment 2 formula II compound
Embodiment 2-1
[0065] Intermediate 1 (compound of formula I) (31.6 grams, 0.1mol), compound of formula III (compound 3) (18.6 grams, 0.1mol), diisopropylethylamine (13.6 grams, 0.105mol) were added to 250ml of In isopropyl acetate, start stirring, heat up to 85°C, keep warm for 3 hours, cool down to room temperature, pour into 300ml of water, stir for 30 minutes, separate the organic phase, extract the aqueous phase with 200ml of isopropyl acetate once, combine The organic phase was successively washed with 300 ml of water and 300 ml of saturated brine, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure, and half of the isopropyl acetate was evaporated to obtain an isopropyl acetate solution of the compound of formula II (intermediate 2). Slowly drop 300ml of n-heptane into the isopropyl acetate solution, solids precipitate out, stir and crystallize at room temperature overnight, filter to obtain the compound of formula II (inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com