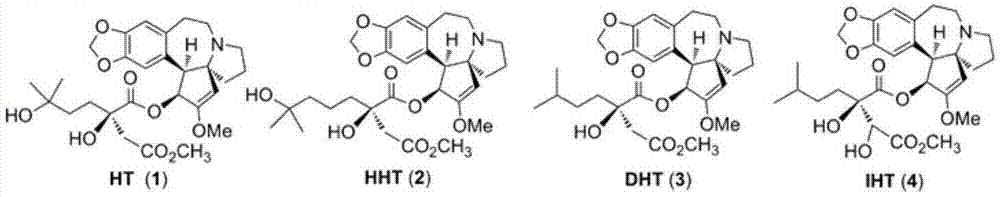
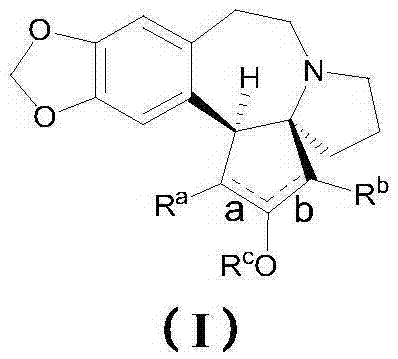
Harringtonines, and preparation method and application thereof
A technology of harringtonine and alkaloids, applied in the field of pharmaceutical compounds and their preparation, in the field of harringtonine alkaloids, and can solve the problems of difficult industrialized production, long synthesis lines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] The preparation of embodiment 1 compound 19
[0183]
[0184] At room temperature, the BF 3 ·OEt 2 85.8μL was added dropwise to 5mL of CH 2 Cl 2 In the solution, the raw material basically disappeared after 5h. Add saturated NaHCO 3 The solution quenched the reaction, after stirring for 0.5h, the organic phase was washed with water until the aqueous phase was not alkaline, washed with saturated NaCl solution, dried, filtered, the solvent was spun off, and column chromatography (PE:EA:TEA=80:10:0.4) Finally, a white solid of compound 19 was obtained with a yield of 87%. mp.139-141℃; [α] D =-170.2° (c 1.03, CHCl 3 ,20℃); 1 H NMR (400MHz, CDCl 3 )δ6.62(s,1H),6.61(s,1H),6.07(s,1H),5.90(s,1H),5.89(s,1H),4.66(d,J=1.8Hz,1H), 3.98(s,1H),3.81(s,1H),3.67(s,3H),3.59(s,3H),3.29(ddd,J=13.8,12.6,7.8Hz,1H),3.05–2.90(m, 2H), 2.98(d, J=16.5Hz, 1H), 2.86–2.80(m, 1H), 2.75(d, J=16.5Hz, 1H), 2.63–2.54(m, 1H), 2.41(dd,J =14.4,7.8Hz,1H),2.28–2.22(m,1H),1.75–1.65(m,4H),1.55–1.3...
Embodiment 2
[0185] The preparation of embodiment 2 compound 3 and 20
[0186]
[0187] Same as Example 1, except that the reaction is carried out at a temperature ranging from -40°C to -78°C. The dr value was C2'R:C2'S=6:1, and compound 3 and compound 20 were obtained after column chromatography (PE:EA:TEA=60:10:0.3). Yield 90%.
[0188] Compound 3: Light yellow amorphous substance, the yield is 75%. [α] D =-122.6° (c 1.03, CHCl 3 ,20℃); 1 H NMR (400MHz, CDCl 3 )δ6.62(s,1H),6.53(s,1H),5.99(d,J=9.8Hz,1H),5.87(d,J=1.5Hz,1H),5.85(d,J=1.2Hz, 1H), 5.04(s, 1H), 3.77(d, J=9.8Hz, 1H), 3.67(s, 3H), 3.57(s, 3H), 3.48(s, 1H), 3.18–3.08(m, 2H ),2.92(td,J=11.0,6.8Hz,1H),2.61–2.57(m,2H),2.36(dd,J=14.0,6.8Hz,1H),2.26(d,J=16.5Hz,1H) ,2.08–1.98(m,1H),1.95–1.87(m,1H),1.88(d,J=16.5Hz,1H),1.77–1.72(m,2H),1.45–1.40(m,3H),1.32 –1.24(m,1H),1.03–0.93(m,1H),0.83(d,J=6.2Hz,3H),0.82(d,J=6.2Hz,3H)ppm; 13 C NMR (101MHz, CDCl 3 )δ 174.2, 170.6, 157.9, 146.7, 145.9, 133.4, 128.5, 112.7, 109.8, 100.9, 100....
Embodiment 3
[0190] The preparation of embodiment 3 compound 19 and 3
[0191]
[0192] Same as Example 1, except that Compound 19 and Compound 3 were obtained from Compound 18 at a temperature range of 0-30°C. , after column chromatography (PE:EA:TEA=80:10:0.4), compound 19 was obtained as a white solid with a yield of 60%, and compound 3 was obtained with a yield of 20%. The products obtained are in agreement with the data for the products of Examples 1 and 2, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com