Ratiometric fluorescent probe for detecting avidin, and synthesis method and application thereof
A technology of avidin and fluorescent probes, which is applied in the field of synthesis of fluorescent probes, can solve the problems of single fluorescence enhancement and rare ratiometric fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
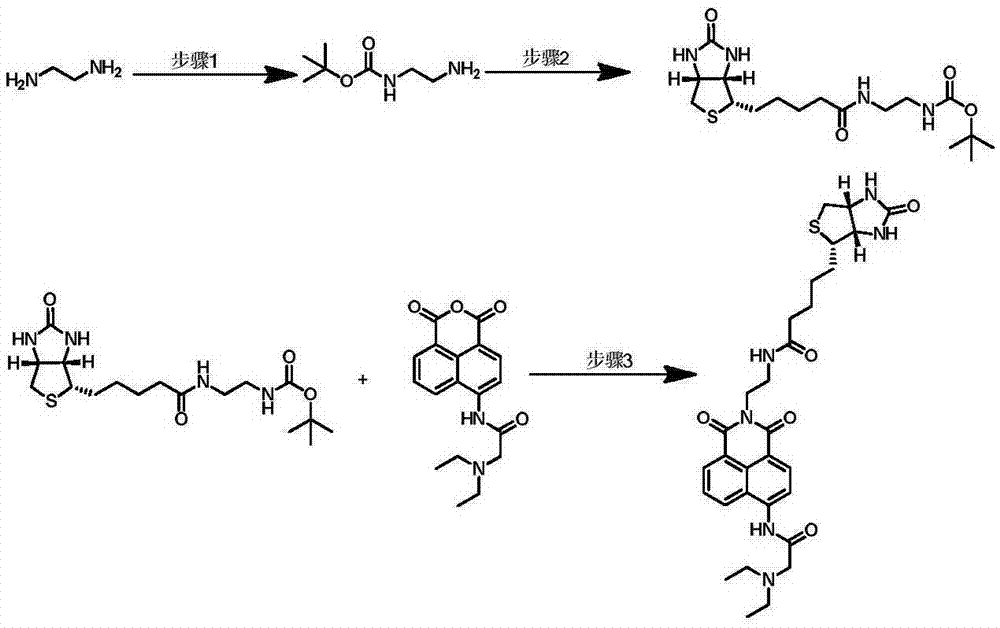
[0030] Example 1: A method for synthesizing a ratiometric fluorescent probe for detecting avidin.
[0031] (1) Synthesis of intermediate N-Boc-ethylenediamine:
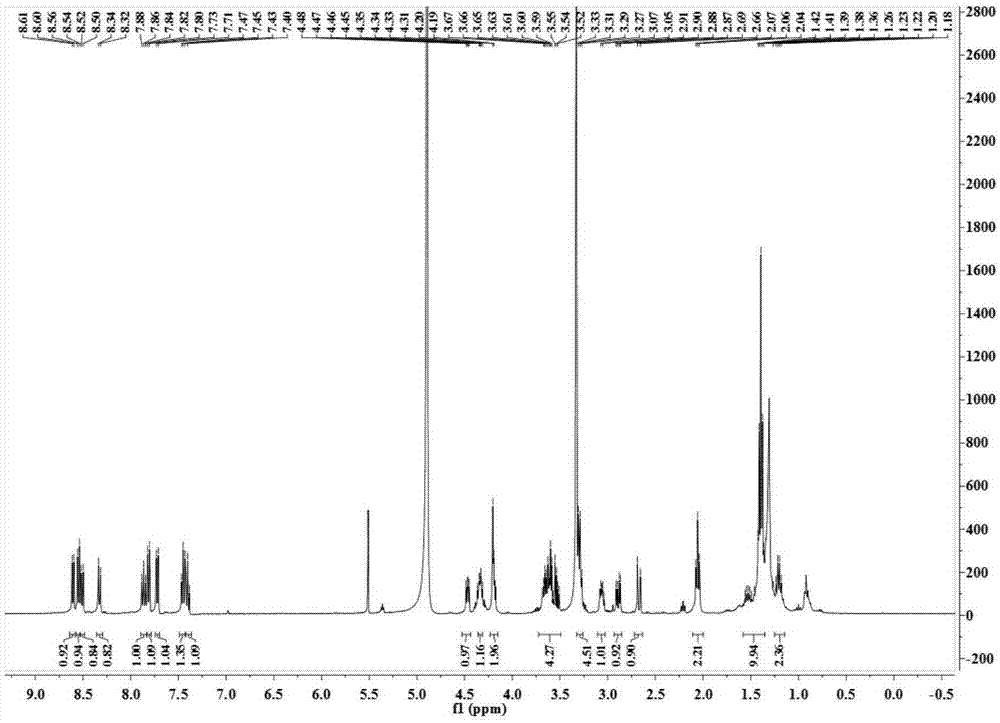
[0032] Di-tert-butyl dicarbonate (1.2 g, 5.5 mmol) was dissolved in 40 mL of dichloromethane, and 2.2 mL (33 mmol) of ethylenediamine was added dropwise under ice-cooling. The reaction solution was stirred at room temperature overnight (10-16 h), washed three times with saturated brine, and the organic phase was dried over anhydrous sodium sulfate, filtered to obtain a clear solution, and spin-dried to obtain 845 mg of a colorless oil, with a yield of 96%. 1 HNMR (400MHz, CDCl 3 ) δ 4.91 (s, 1H), 3.20–3.14 (m, 2H), 2.80 (t, J=5.9Hz, 2H), 1.45 (s, 9H), 1.41 (s, 2H).
[0033]
[0034] (2) Synthesis of intermediate N-Boc-N-biotin ethylenediamine:
[0035] EDC.HCl (144mg, 1.0mmol), HOBt.H2O (182mg, 1.0mmol), triethylamine (350μL, 2.1mmol), biotin (110mg, 0.2mmol) were dissolved in 10mL DMF, and N -Boc-Ethylenediami...
Embodiment 2
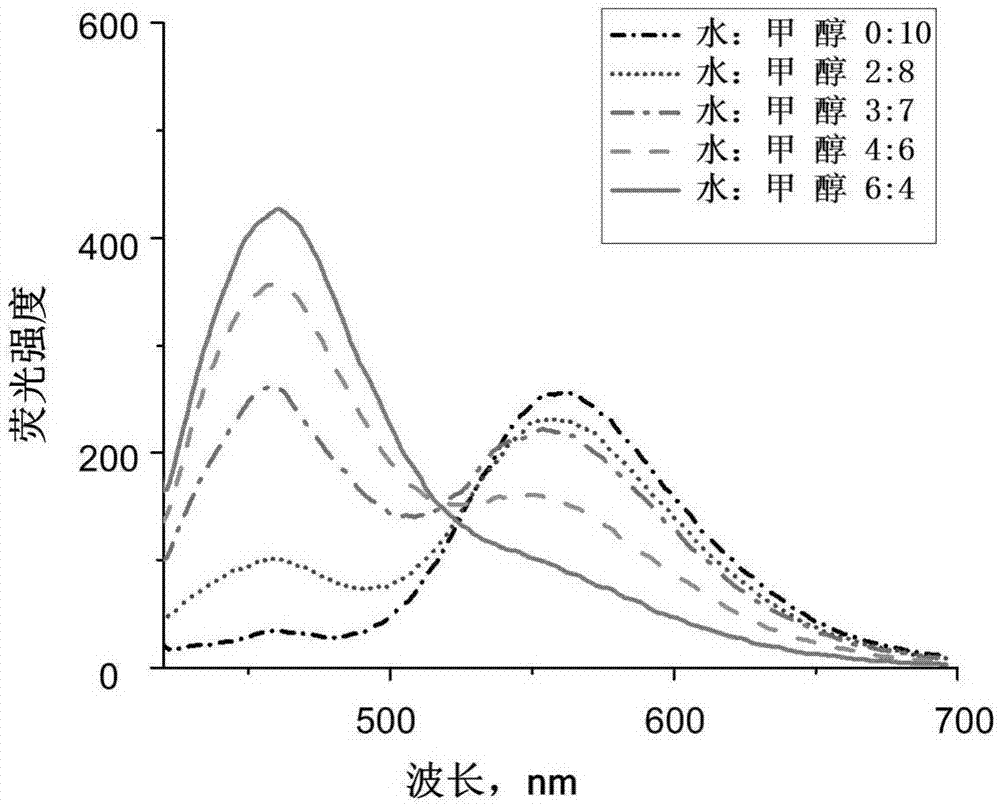
[0041] Example 2: Fluorescent response of the fluorescent probe prepared in Example 1 under different ratios of water and methanol.
[0042] image 3 The concentration of the fluorescent probe prepared in Example 1 is 10 μM, and the excitation light is 405 nm. The probe exhibits fluorescence with a wavelength of ~470 nm in aqueous solution, and fluorescence with a wavelength of ~550 nm in methanol. When the proportion of methanol gradually increases, the wavelength The fluorescence intensity at ~550nm increases gradually, while the fluorescence intensity at ~470nm decreases gradually.
Embodiment 3
[0043] Example 3: The response of the fluorescent probe prepared in Example 1 to 0-2.0 equivalents of avidin.
[0044] Figure 4 The fluorescent probe concentration in the medium is 5 μM, the excitation light is 365 nm, 10.0 μL of the probe mother solution is dissolved in 4 mL of PBS buffer (pH=7.4, 20 mM), and a test solution with a fluorescent probe concentration of 5 μM is prepared. When the test solution is gradually added relative to the probe 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5, 2.0 equivalents of avidin (0, 1.0, 2.0, 3.0, 4.0 , 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 15.0, 20.0μL), the fluorescence intensity at ~470nm gradually decreased, while the fluorescence at ~550nm gradually increased.
[0045] Figure 5 The fluorescent probe concentration in the medium is 5 μM, the excitation light is 405 nm, 10.0 μL of the probe mother solution is dissolved in 4 mL of PBS buffer (pH=7.4, 20 mM), and a test solution with a fluorescent probe concentration of 5 μM is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com