A kind of synthetic method of methylpiperium former medicine
A synthesis method and mepinium technology are applied in the field of synthesis of mepinium original medicine, and achieve the effects of high purity, simple process operation, significant economic benefits and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
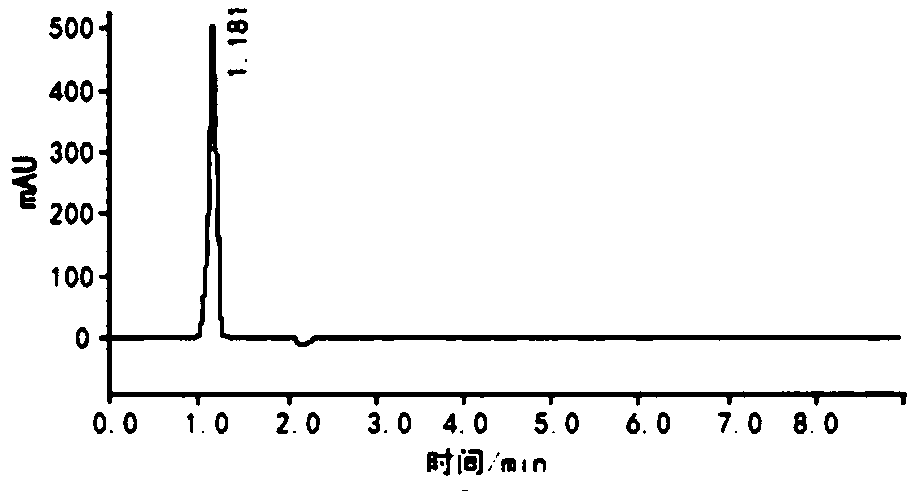
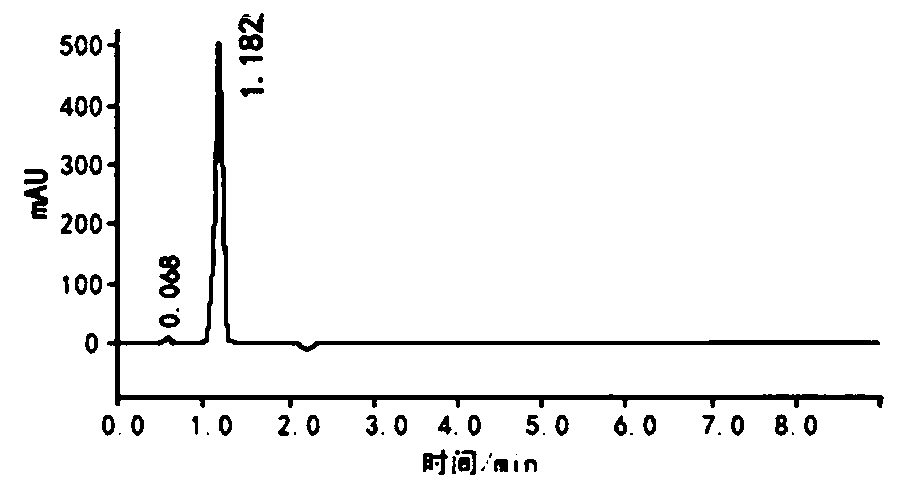
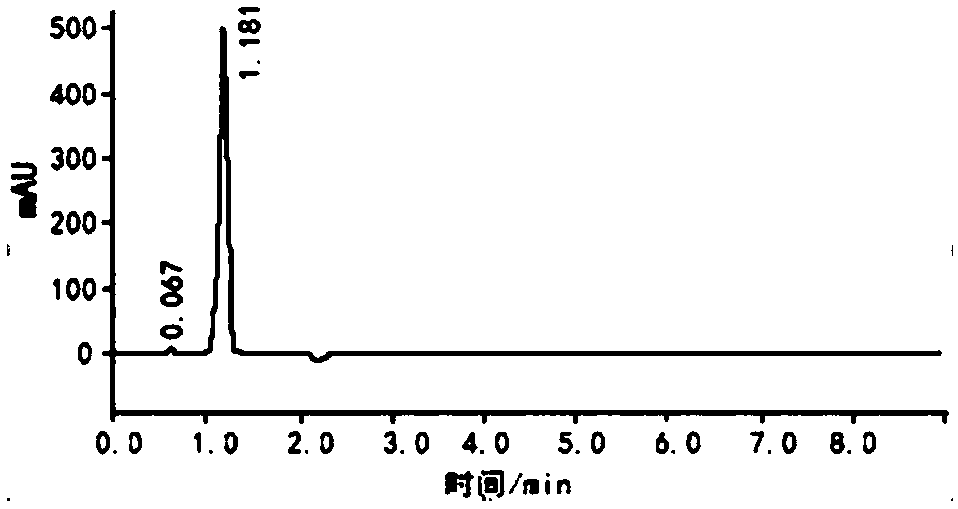
Image
Examples
Embodiment 1
[0038] The synthetic method of the former medicine of methylpiperium of the present invention, the detailed steps of this synthetic method are as follows:
[0039] a. First, add ethanol with a mass percentage concentration of 99.5% into the reaction vessel, then add the raw material pyridine for mixing, and add methyl chloride after dissolving; then heat up to 80°C, and control the pressure during the reaction to 0.5MPa. 1. Reaction under pressure conditions for 6h, and N-picoline chloride was obtained after the reaction;
[0040] The mass ratio of the amount added between the pyridine and ethanol is 1:2.5; the molar ratio of the amount added between the pyridine and methyl chloride is 1:1.2;
[0041] B, adding the N-picoline chloride obtained in step a and the bimetallic supported catalyst Ru-Pd / C in the reaction vessel, the addition of the catalyst accounts for 4% of the N-picoline chloride quality; then Pass hydrogen into the reactor, the molar ratio between the N-picoline...
Embodiment 2
[0047] The synthetic method of the former medicine of methylpiperium of the present invention, the detailed steps of this synthetic method are as follows:
[0048] a. First, add ethanol with a mass percentage concentration of 97% into the reaction vessel, then add the raw material pyridine for mixing, and add methyl chloride after dissolving; then heat up to 100°C, and control the pressure during the reaction to 0.7MPa. 1. Reaction under pressure conditions for 4h, and N-picoline chloride was obtained after the reaction;
[0049]The mass ratio of the amount added between the pyridine and ethanol is 1:3.5; the molar ratio of the amount added between the pyridine and methyl chloride is 1:1.1;
[0050] B, the N-picoline chloride that step a obtains and PTP-20 pyridine hydrogenation catalyst are added in the reaction vessel, the addition of described catalyst accounts for 3% of N-picoline chloride quality; Then to reactor Introduce hydrogen gas, the molar ratio between the N-pico...
Embodiment 3
[0056] The synthetic method of the former medicine of methylpiperium of the present invention, the detailed steps of this synthetic method are as follows:
[0057] a. First, add ethanol with a mass percentage concentration of 95% into the reaction vessel, then add the raw material pyridine for mixing, and add methyl chloride after dissolving; then heat up to 60°C, and control the pressure during the reaction to 0.9MPa. 1. Reaction under pressure conditions for 6h, and N-picoline chloride was obtained after the reaction;
[0058] The mass ratio of the amount added between the pyridine and ethanol is 1:5; the molar ratio of the amount added between the pyridine and methyl chloride is 1:1.2;
[0059] B, adding the N-picoline chloride obtained in step a and the bimetallic supported catalyst Ru-Pd / C into the reaction vessel, the addition of the catalyst accounts for 5% of the N-picoline chloride quality; then Pass hydrogen into the reactor, the molar ratio between the N-picoline c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com