Novel method for separation of Gram-negative pathogenic bacteria in sepsis
A gram-negative, separation method technology, applied in the field of gram-negative pathogenic bacteria separation based on magnetic nanoparticles, can solve the problems of long cycle time, manpower and material resources, etc., and achieve the effect of low cost, long shelf life and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
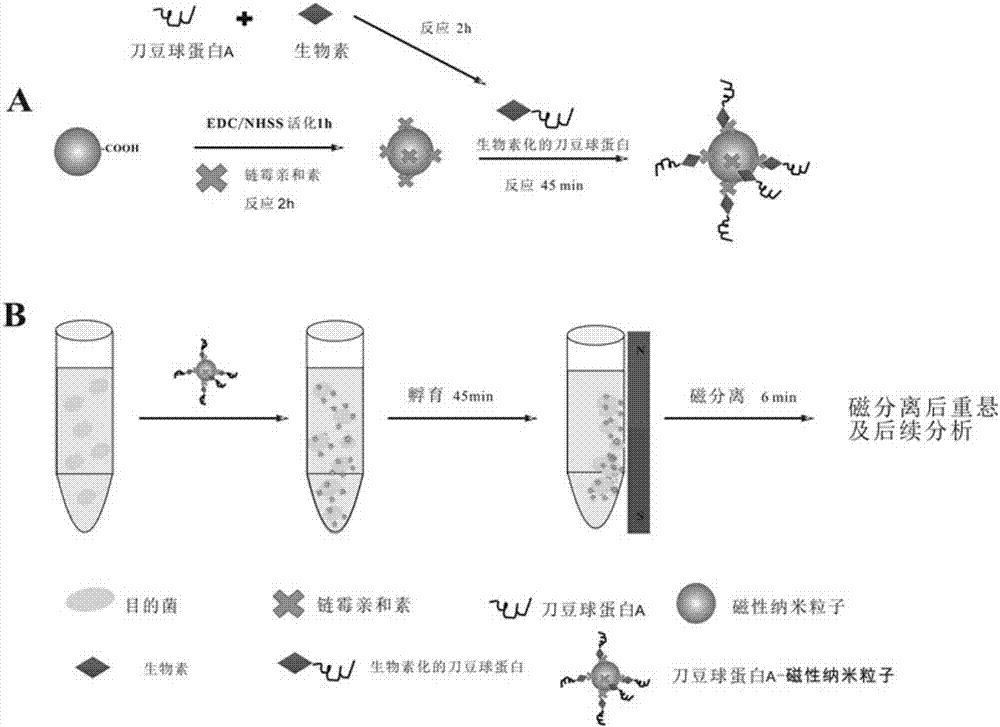
[0027] 1. Concanavalin A-magnetic nanoparticle complex is synthesized, prepared according to the following steps:
[0028](1) Draw 2mL of magnetic nanoparticles (10mg / mL), add them to 8mL of sterile PBS solution with pH=7.4, and then wash the magnetic nanoparticles under the action of an external magnetic field, repeat 3 times, and wash The final magnetic nanoparticles were resuspended in 10 mL sterile PBS solution; (2) 5.8 mg EDC was dissolved in 290 μL sterile PBS, 6.5 mg NHSS was dissolved in 325 μL sterile PBS, and then the dissolved EDC and NHSS were added to the washed magnetic nanoparticles solution and activated for 1 h; (3) the activated magnetic nanoparticles were washed 3 times with sterile PBS and resuspended in 8 mL of sterile PBS solution; (4) weighed Take streptavidin 0.5 mg, dissolve it in 100 μL sterilized PBS solution, then add it into the activated magnetic bead solution, react for 2 hours, then wash 3 times with sterilized PBS, resuspend in 8 mL sterilized ...
Embodiment 2
[0031] Embodiment 2 enrichment effect experiment
[0032] (1) Take 1mL concentration as 10 5 CFU / mL Gram-negative pathogenic bacteria were placed in a 1.5mL sterile centrifuge tube, centrifuged at 12000rpm for 5min, discarded the supernatant, and resuspended with an equal volume of sterile PBS solution.
[0033] (2) Enrichment and capture: Take 10 mL of the sample solution to be tested, add 1 mg of concanavalin A-magnetic nanoparticle complex, place it on a mixer, and incubate at room temperature for 45 minutes at a speed of 200 rpm to form a Gram-negative pathogen-Concanavalin Protein A-magnetic nanoparticle complex; insert the centrifuge tube into a conventional magnetic stand for separation for 6 minutes;
[0034] (3) After magnetic separation, the supernatant was transferred to a sterile centrifuge tube, and the isolated Gram-negative pathogen-concanavalin A-magnetic nanoparticle complex was washed twice with PBS, mixed evenly, and Resuspend the Gram-negative pathogen-co...
Embodiment 3
[0040] The sample to be tested is sterile lysed blood, and Gram-negative pathogenic bacteria are added to adjust the colony concentration to 10 5 CFU / mL for use.
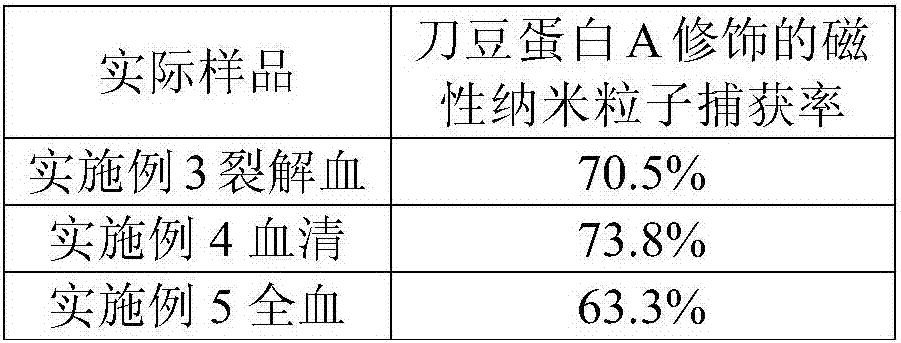
[0041] The prepared concanavalin A-magnetic nanoparticle complex (1 mg) was added to the sample solution (10 mL), placed on a mixer, and incubated at room temperature for 45 min at a speed of 200 rpm. Then the conventional magnetic stand was separated for 6 minutes. After magnetic separation, the supernatant was transferred into a sterile centrifuge tube, and the isolated Gram-negative pathogen-concanavalin A-magnetic nanoparticle complex was washed twice with sterile PBS, mixed evenly, and washed with 1 mL Resuspend the Gram-negative pathogen-concanavalin A-magnetic nanoparticle complex in sterile PBS solution. Capture efficiency is obtained as in Example 2, and the rest are the same as in Example 2. The results are shown in Table 1, indicating that this protocol can efficiently enrich the Gram-negative pathogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com