Amorphous nanospherical active ferric phosphate hydrate, and preparation method and application thereof
An amorphous, iron phosphate technology, applied in phosphorus compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of long reaction time, limited industrial application, poor particle size uniformity of spherical iron phosphate, etc., and achieve the effect of improving electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
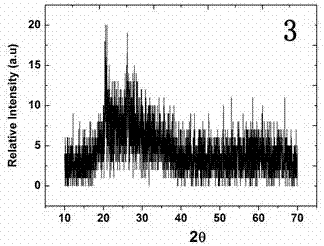
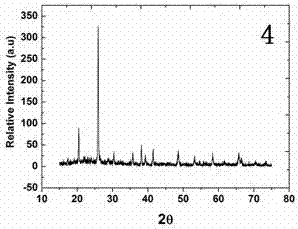
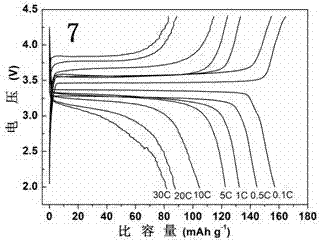
Image
Examples
Embodiment 1
[0053] 1. To prepare amorphous nano-spherical active iron phosphate hydrate, the following steps are adopted:
[0054] (1) Weigh 2 grams of urea, dissolve it in 50ml of distilled water, and stir for 30 minutes to obtain solution 1;
[0055] (2) Weigh 1 gram of sodium dodecyl sulfate (SDS), dissolve it in 50ml of distilled water, and stir for 30 minutes to obtain solution 2;
[0056] (3) Mix solution 1 and solution 2 to obtain solution A;
[0057] (4) Weigh 1.01 g (2.5 mmol) of ferric nitrate nonahydrate as an iron source, dissolve it in 90 ml of distilled water, and stir for 30 minutes to obtain ferric nitrate nonahydrate solution;
[0058] (5) Add the ferric nitrate nonahydrate solution obtained in step (4) dropwise to solution A obtained in step (3), and stir for 30 minutes to obtain solution B;
[0059] (6) Weigh a phosphoric acid solution with a mass concentration of 85%, the molar ratio of phosphoric acid in the phosphoric acid solution to the ferric nitrate nonahydrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com