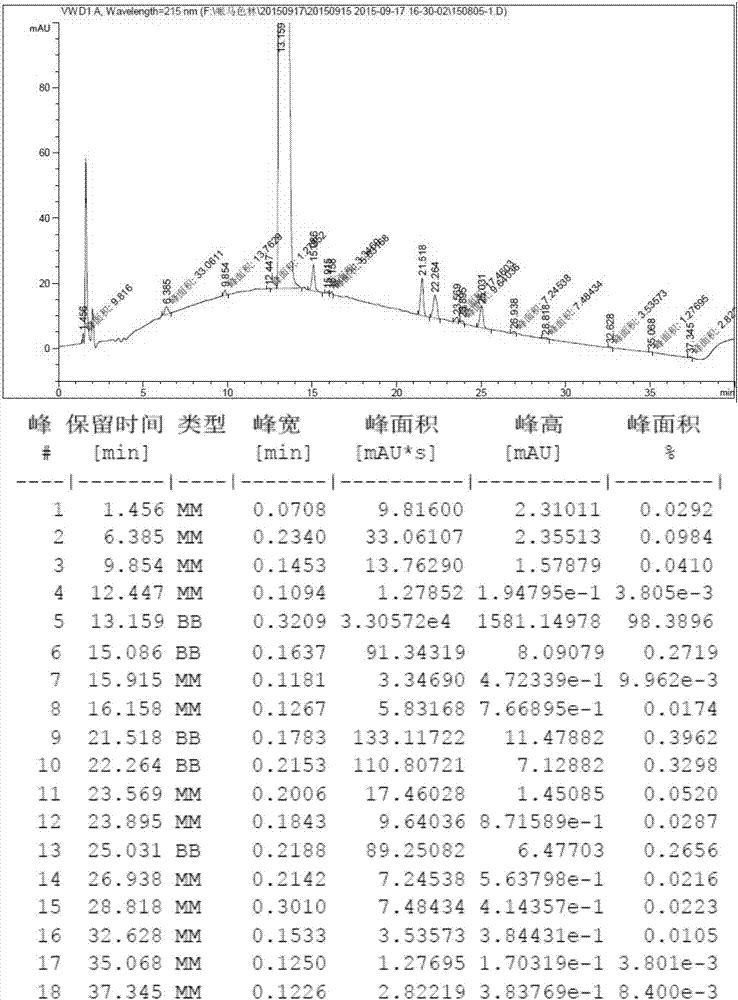
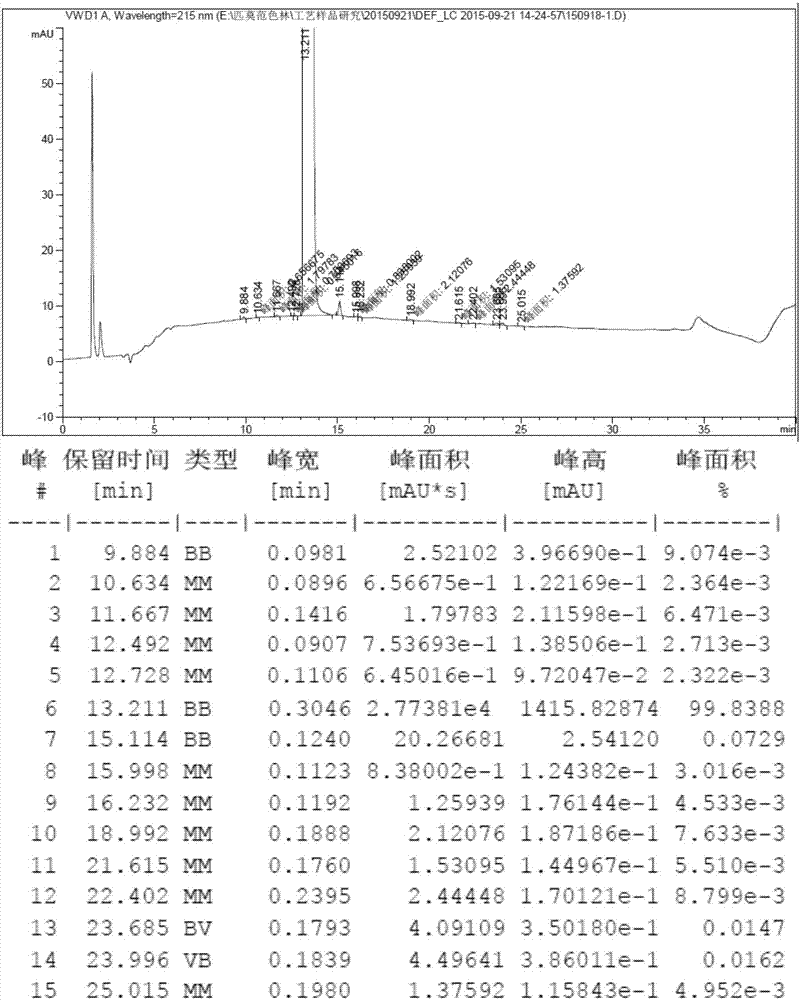
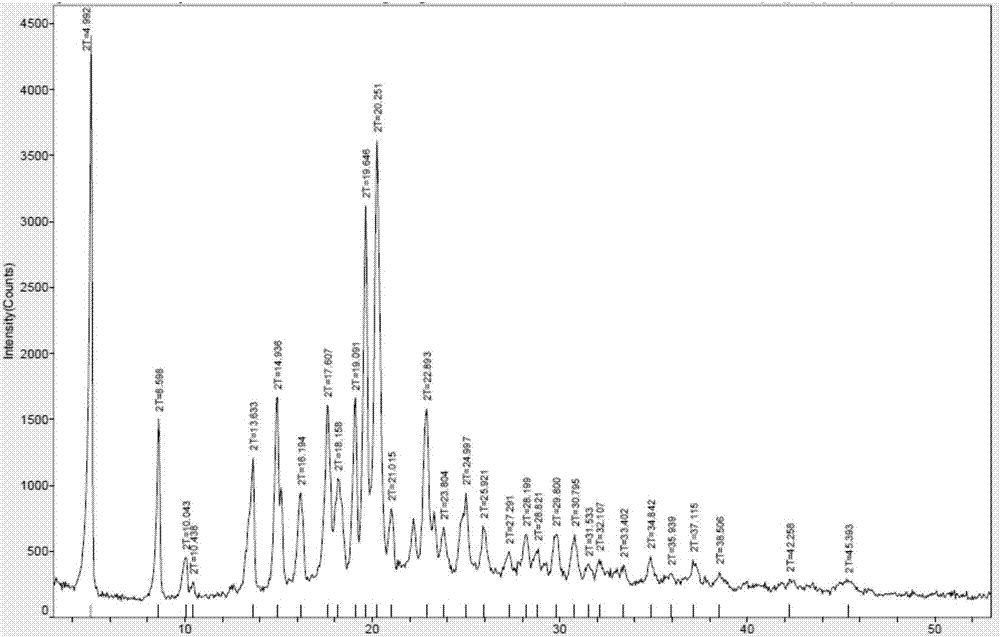
Method for preparing crystal form B of pimavanserin semi-tartrate
A technique for hemi-tartrate and pimaserin, which is applied in the field of preparing the hemi-tartrate crystal form B of pimaserine, can solve problems such as difficulties, and achieves the effects of high production efficiency, good economic benefit and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Pimaserin hemitartrate can be obtained by purchasing commercially available products, or can be prepared by the preparation method of Chinese patent CN102153505 A, and can also be prepared by the following methods:
[0038] 4-isobutoxybenzylamine (17.9g) was dissolved in toluene (73.0g) solution, the toluene solution was cooled to 1-5°C and then hydrogen chloride gas (4.0g) was introduced, keeping the temperature at 10°C, and the , heated to 97-103°C, introduced phosgene (16.2g) through the catheter, after the addition, cooled to 80-85°C, TLC detected that the reaction was complete, stopped the reaction, cooled to room temperature, and obtained a toluene solution of isocyanate.
[0039] At 40°C, the toluene solution of the above isocyanate was added dropwise to a solution of 4-(4-fluorobenzylamino)-1-methylpiperidine (21.7g) in tetrahydrofuran (195g), stirred and reacted for 3 hours, and the reaction was detected by TLC After completion, concentrate under reduced pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com