2-pyrimidinyloxy benzoic acid derivative and preparation method thereof, and herbicide for water weed
A technology of pyrimidinyloxybenzoic acid and derivatives, applied in the field of pesticides, can solve the problems of time-consuming and laborious, easy precipitation, poor water solubility, etc., and achieve high-efficiency herbicidal effect and good water-solubility effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention provides the preparation method of 2-pyrimidinyloxybenzoic acid derivative described in above-mentioned technical scheme, comprises the following steps:
[0056] Mix 2-pyrimidinyloxybenzoic acid compounds, aprotic solvents and organic salts, and carry out a substitution reaction at -10 to 150°C for 0.5 to 48 hours under the action of a catalyst to obtain 2-pyrimidinyloxybenzoic acid derivatives;
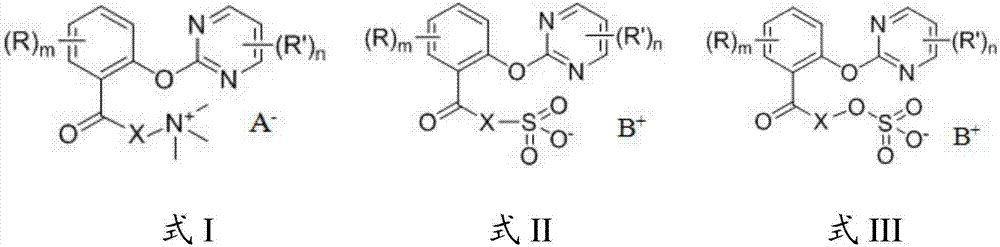
[0057] The organic salt is quaternary ammonium salt, sulfonate or organic sulfate.
[0058] In the present invention, the molar ratio of the 2-pyrimidinyloxybenzoic acid compound, aprotic solvent, catalyst and organic salt is preferably 1: (100-1000): (0.001-2): (1-10), More preferably 1:(200-800):(0.05-1.5):(2-8), most preferably 1:(400-600):(0.5-1):(3-6).
[0059] In the present invention, the selection of the 2-pyrimidinyloxybenzoic acid compound corresponds to the 2-pyrimidinyloxybenzoic acid derivative having the structure shown in formula I, formul...
Embodiment 1
[0084] Mix 2-pyrimidinyloxybenzoic acid (1 mmol), 1-bromo-N,N,N trimethyldodecylammonium bromide (5 mmol), sodium hydroxide (2 mmol) and dimethyl sulfoxide (800 mmol) , at a stirring rate of 500rpm, the substitution reaction was carried out at 120°C for 24h;
[0085] Filter the obtained material, remove the solvent in the obtained filtrate by distillation under reduced pressure, wash with saturated aqueous sodium hydroxide solution, extract with dichloromethane (3×20 mL), wash with water, and dry with anhydrous sodium sulfate to obtain a dry material;
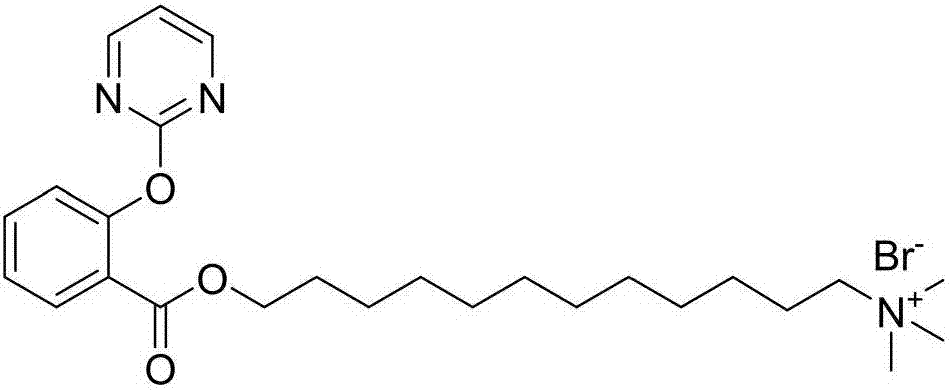
[0086] The solvent in the obtained dry material was distilled off under reduced pressure, and a mixed solvent of ethyl acetate and petroleum ether was used as an eluent (the volume ratio of ethyl acetate and petroleum ether was 1:1), and separated by column chromatography to obtain a light yellow liquid , which is N,N,N-trimethyl-12-((2-(pyrimidine-2-oxy)benzaldehyde)oxy)dodecyl-1-ammonium bromide, the structure is as follows: ...
Embodiment 2
[0092] (4-methoxy)-2-pyrimidinyloxy-5-chlorobenzoic acid (1mmol), 1-bromo-N,N,Ntrimethyldodecylammonium bromide (5mmol), potassium hydroxide ( 2mmol) and dimethyl sulfoxide (800mmol) were mixed, and at a stirring rate of 400rpm, a substitution reaction was carried out at 120°C for 24h;
[0093] Filter the obtained material, remove the solvent in the obtained filtrate by distillation under reduced pressure, wash with saturated aqueous sodium hydroxide solution, extract with dichloromethane (3×20 mL), wash with water, and dry with anhydrous sodium sulfate to obtain a dry material;
[0094] The solvent in the obtained dried material was distilled off under reduced pressure, and a mixed solvent of ethyl acetate and petroleum ether was used as an eluent (the volume ratio of ethyl acetate and petroleum ether was 1:1.2), and separated by column chromatography to obtain a light yellow liquid , which is 12-((5-chloro-2-((4-methoxypyrimidin-2-yl)oxy)benzoyl)oxy)-N,N,N-trimethyldodecyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com