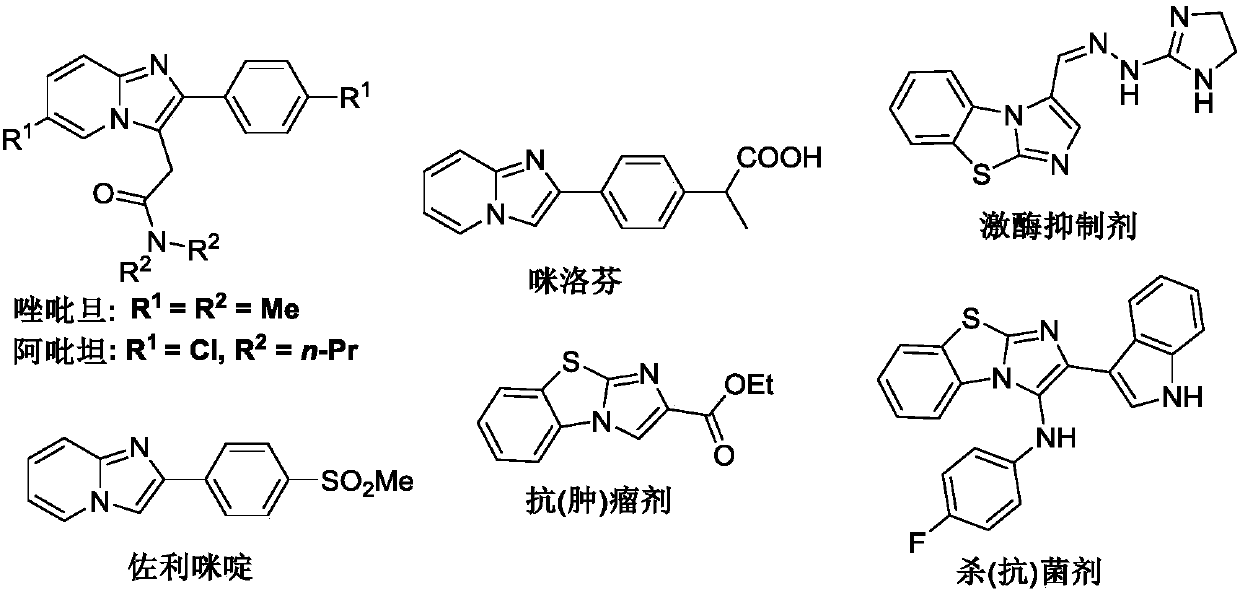
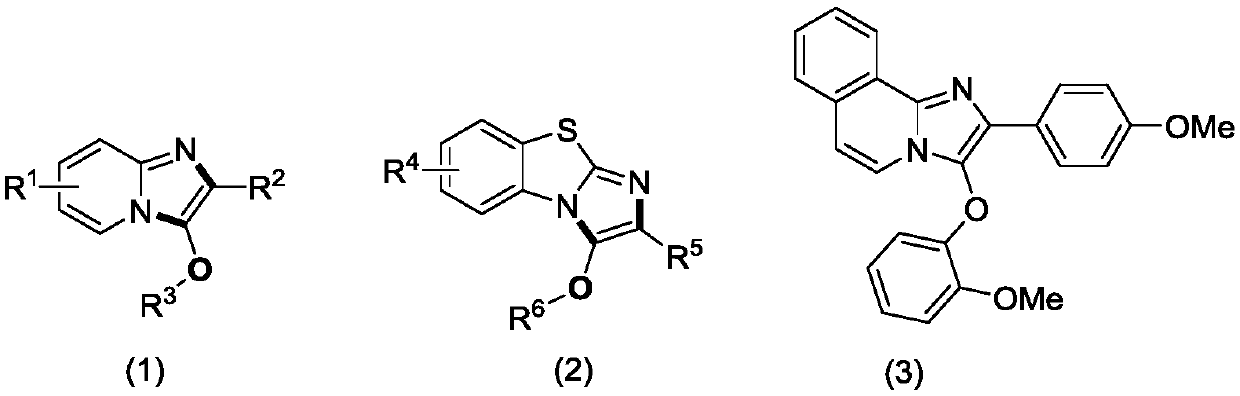
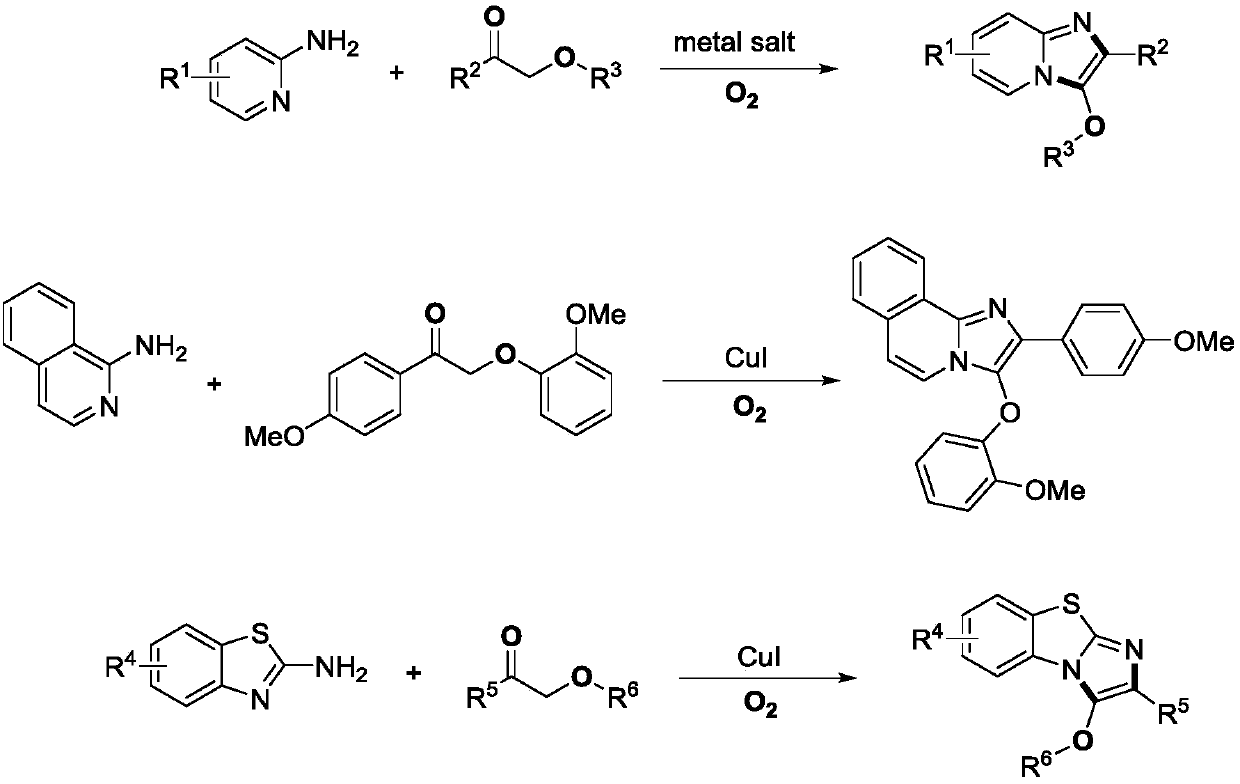
A kind of preparation method of imidazole heterocyclic compound substituted by C-3 position oxygen
A technology of compounds and synthetic methods, applied in the directions of organic chemistry, drug combination, anti-tumor drugs, etc., to achieve the effects of high economic benefit, good economic value and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0032]
[0033] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01mmol) , 1,2-dichloroethane (1.0 mL), heated at 100° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (44.0 mg, yield 77%). White solid, m.p.:145-146℃. 1 H NMR (400MHz, CDCl 3 ): δ=8.07(d, J=7.6Hz, 2H), 7.75(d, J=6.8Hz, 1H), 7.66(d, J=9.2Hz, 1H), 7.41(t, J=7.2Hz, 2H ),7.28-7.35(m,3H),7.20(t,J=7.6Hz,1H),7.12(t,J=7.2Hz,1H),6.97(d,J=8.0Hz,2H),6.77(t ,J=6.8Hz,1H). 13 C NMR (100MHz, CDCl 3 ):δ=156.1,139.9,132.5,131.1,130.2,128.7,127.8,126.5,124.4,123.7,121.6,117.9,115.0,112.3.FTIR(NaCl,cm -1 ):3053.3,2985.8,1647.2,1495.6,1421.5,1390.1,1362.2,1265.3,1201.7,895.0,738.7,706.0.HR-MS(ESI):m / zcalculated for C 19 h 14 N 2 O[M+H]+: 287.1184; found 287.1...
Embodiment 2
[0034] Example 2: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0035]
[0036] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01mmol) , 1,2-dichloroethane (1.0 mL), heated at 80° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (40.0 mg, yield 70%).
Embodiment 3
[0037] Example 3: Preparation of 3-phenoxy-2-phenylimidazo[1,2-a]pyridine
[0038]
[0039] Take a clean reaction bottle, add a small magnet, dry it, add 2-aminopyridine (56.5mg, 0.6mmol), 2-phenoxyacetophenone (42.5mg, 0.2mmol), cuprous iodide (0.01mmol) , 1,2-dichloroethane (1.0 mL), heated at 120° C. under oxygen for 16 hours, and the reaction solution was separated by direct column chromatography to obtain the target product (37.8 mg, yield 66%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com