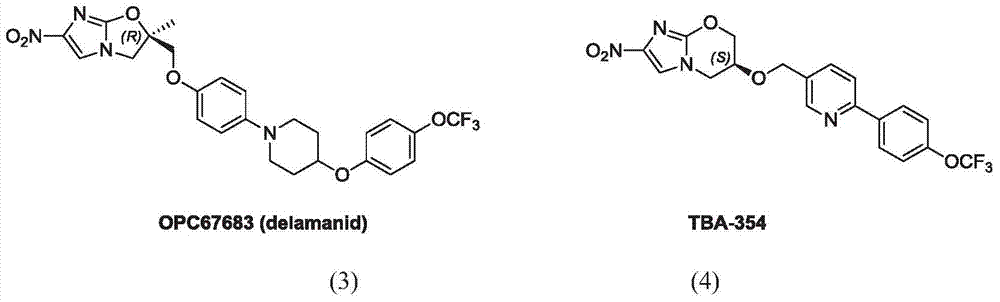
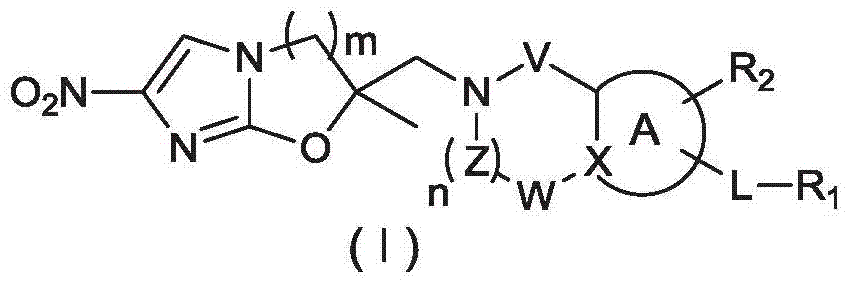
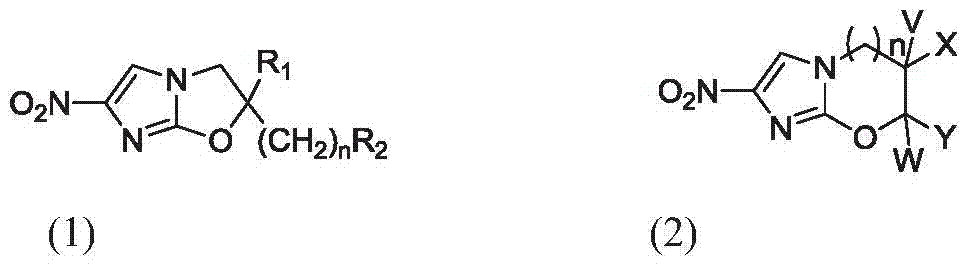Anti-pulmonary tuberculosis nitroimidazole derivative
An alkyl and cyano technology, applied in the field of diseases caused by drug resistance combined with mycobacteria, can solve problems such as low activity and high side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] 2-methyl-6-nitro-2-((2-phenyl-7,8-dihydro-1,6-naphthyridin-6(5H)-yl)methyl)-2,3-dihydro Imidazo[2,1-B]oxazole
[0215]
[0216] step 1:
[0217] 6-Benzyl-2-phenyl-5,6,7,8-tetrahydro-1,6-naphthalene
[0218]
[0219] To the key intermediate A (1.10 g, 4.25 mmol) in dioxane / water (11 ml, 10 / 1) mixed solution was added potassium phosphate (2.23 g, 10.51 mmol), phenylboronic acid (1.03 g) , 8.45 mmol) and Pd (PPh 3 ) 4 (734.00 mg, 635.19 micromolar), the mixture was stirred at 130 degrees Celsius for 3 hours. The mixture was filtered, the filtrate was concentrated, and the residue was purified by silica gel chromatography (petroleum ether / ethyl acetate=10 / 0, 10 / 1) to obtain 6-benzyl-2-phenyl-7,8-dihydro-5H -1,6-Naphthalene (750.00 mg, 58.75%), a white solid. H NMR(400MHz, CDCl 3 ): δ7.96(d, J=7.2Hz, 2H), 7.53-7.36(m, 10H), 3.76(s, 2H), 3.69(s, 2H), 3.16(t, J=5.9Hz, 2H) , 2.93 (t, J = 5.9 Hz, 2H). LCMS (ESI) m / z: 301 (M+1). LCMS (ESI) m / z: 301 (M+1).
[0220] Step 2:
[0221] 2-...
Embodiment 2
[0233] 2-methyl-6-nitro-2-((2-(4-(trifluoromethoxy)phenyl)-7,8-dihydro-1,6-naphthyridin-6(5H)-yl )Methyl)-2,3-dihydro[2,1-B]oxazole
[0234]
[0235] step 1:
[0236] 6-Benzyl-2-(4-(trifluoromethoxy)phenyl)-5,6,7,8-tetrahydro-1,6-naphthalene
[0237]
[0238] To a mixed solution of key intermediate A (1.20 g, 4.64 mmol, 1.00 equivalent) in dioxane (5 mL) and water (0.5 mL) was added potassium phosphate (2.46 g, 11.60 mmol, 2.50 equivalent), Add Pd(PPh 3 ) 4 (536.18 mg, 464.00 micromole, 0.10 equivalent), (4-(trifluoromethoxy)phenyl)boronic acid (1.43 g, 6.96 mmol, 1.50 equivalent). The mixture was stirred at 120 degrees Celsius for 3 hours. The mixture was cooled and concentrated under reduced pressure at 50 degrees Celsius. The residue was purified by silica gel chromatography (petroleum ether / ethyl acetate=50 / 1, 15 / 1) to obtain 6-benzyl-2-(4-(tri (Fluoromethoxy)phenyl)-5,6,7,8-tetrahydro-1,6-naphthalene (1.30 g, 3.38 mmol, 72.89% yield) as a yellow solid. LCMS(ESI) m / z: 385(M+...
Embodiment 3
[0252] (S)-2-Methyl-6-nitro-2-((2-(3-(trifluoromethoxy)phenyl)-7,8-dihydro-1,6-naphthyridine-6( 5H)-yl)methyl)-2-,3-dihydroimidazo[2,1-B]oxazole
[0253]
[0254] The key intermediate B (120.00 mg, 343.08 micromole, 1.00 equivalent) and [3-(trifluoromethoxy)phenyl]boronic acid (70.65 mg, 343.08 micromole, 1.00 equivalent) were dissolved in dioxane (5.00 Ml) and water (500.00 microliters), the solution was added Pd(dppf)Cl at 30 degrees Celsius under nitrogen protection 2 (25.10 mg, 34.31 micromole, 0.10 equivalent), sodium carbonate (90, 91 mg, 857.70 micromole, 2.5 equivalent). The mixture was stirred at 110 degrees Celsius for 12 hours. After the reaction, the mixture was cooled to 30 degrees Celsius and concentrated under reduced pressure at 45 degrees Celsius. The residue was poured into water (10 mL) and stirred for 5 minutes. The aqueous phase was extracted with ethyl acetate (30 mL×3). The combined organic phase was washed with saturated brine (10 mL×2), dried over anh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com