Tyrosinase inhibitor, preparation method and uses thereof
A tyrosinase and inhibitor technology, applied in the field of hydroxypyridone derivatives, can solve problems such as the effect of inhibiting enzyme activity, and achieve the effects of good stability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
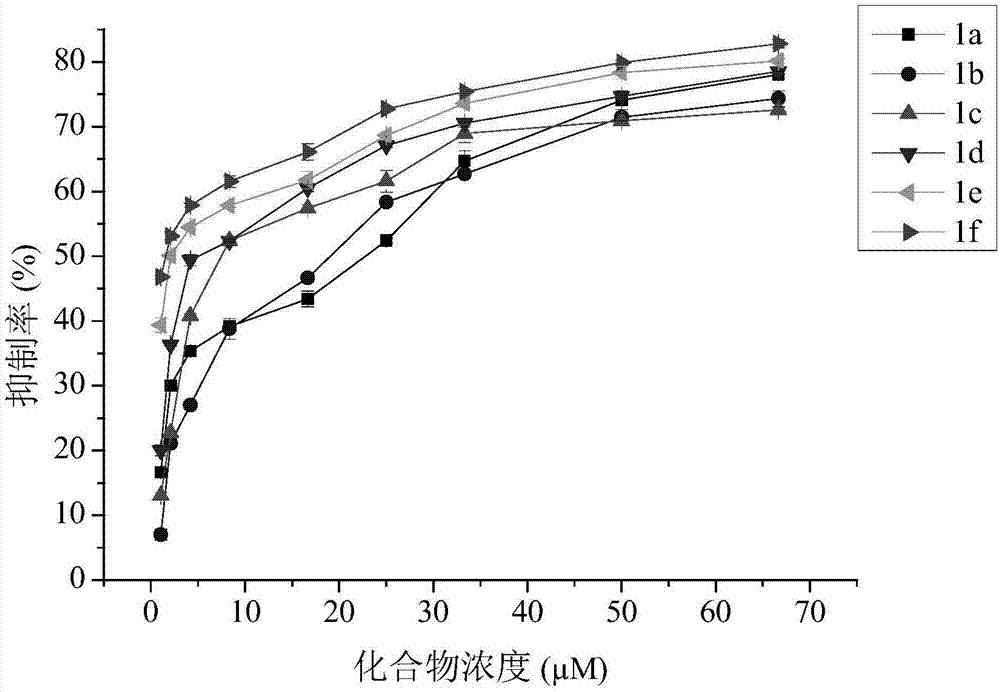
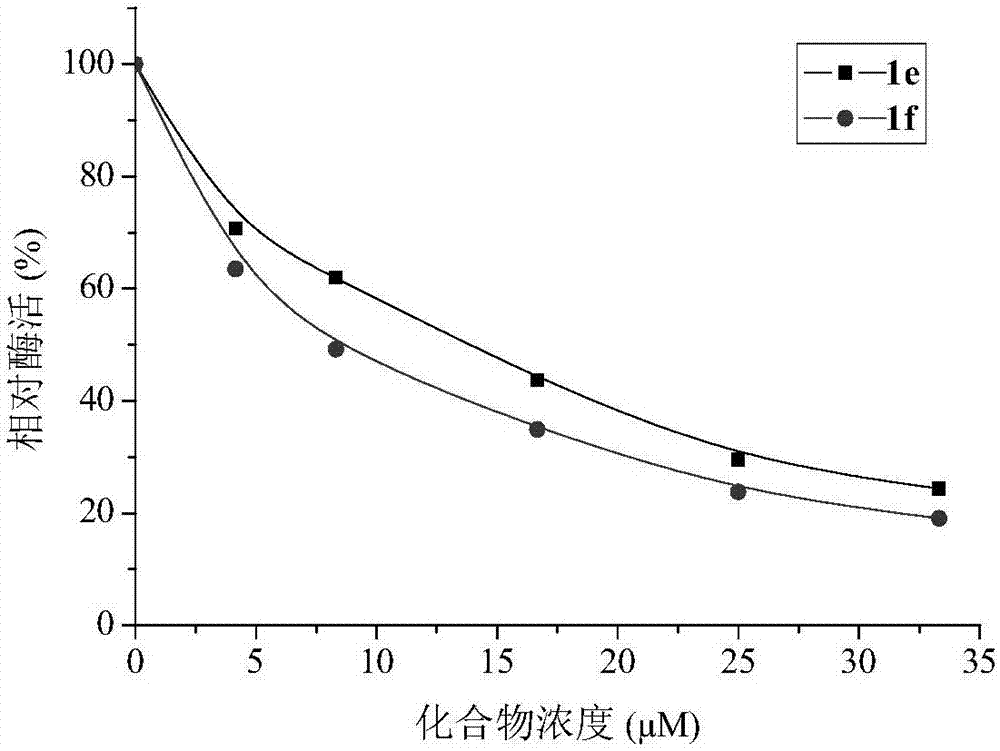
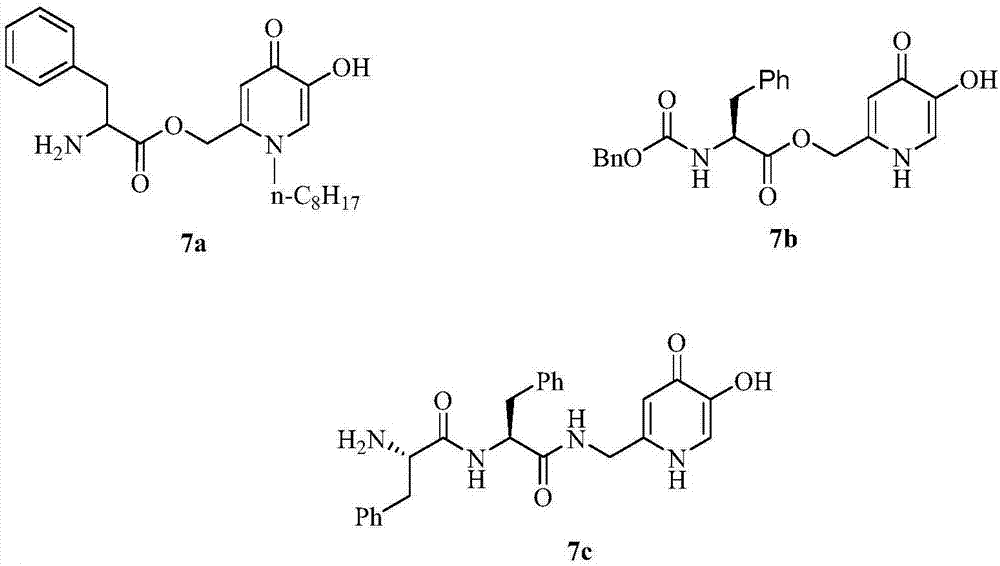
[0024] Embodiment 1, the synthesis of compound 1a-1f:
[0025] The routes for the synthesis of compounds 1a-1f from kojic acid are shown in Scheme 1.
[0026]
[0027] Compound 3 was synthesized from kojic acid (2) according to the method reported in the literature (Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. Journal of Agricultural and Food Chemistry 2013, 61(27), 6597-6603) .
[0028] A, the synthesis of compound 4
[0029] 1), the synthesis of compound 4a-4c
[0030] Take 10 g of compound 3 and add 17 mL of absolute ethanol, heat and reflux in an oil bath while stirring, then measure 83 mL of ammonia water (mass concentration 25-28%) into it, react at 60°C overnight, and monitor the reaction progress by TLC. After the reaction was completed (reaction time was 12 hours), it was cooled to room temperature and left to stand. The precipitate was collected by filtration, washed twice with a small amount of ether,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com